Page 250 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 250
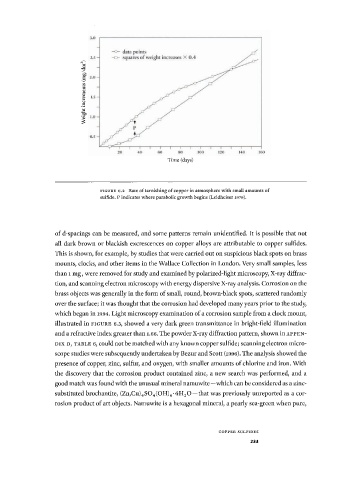
Time (days)
F I G U R E 6 . 2 Rate of tarnishing of copper in atmosphere with small amounts of
sulfide. Ρ indicates where parabolic growth begins (Leidheiser 1979) .
of d-spacings can be measured, and some patterns remain unidentified. It is possible that not
all dark brown or blackish excrescences on copper alloys are attributable to copper sulfides.
This is shown, for example, by studies that were carried out on suspicious black spots on brass
mounts, clocks, and other items in the Wallace Collection in London. Very small samples, less
than 1 mg, were removed for study and examined by polarized-light microscopy, X-ray diffrac
tion, and scanning electron microscopy with energy dispersive X-ray analysis. Corrosion on the
brass objects was generally in the form of small, round, brown-black spots, scattered randomly
over the surface; it was thought that the corrosion had developed many years prior to the study,
which began in 1994. Light microscopy examination of a corrosion sample from a clock mount,
illustrated in FIGURE 6.3, showed a very dark green transmittance in bright-field illumination
and a refractive index greater than 1.66. The powder X-ray diffraction pattern, shown in APPEN
DIX D, TABLE 6, could not be matched with any known copper sulfide; scanning electron micro
scope studies were subsequently undertaken by Bezur and Scott (i996). The analysis showed the
presence of copper, zinc, sulfur, and oxygen, with smaller amounts of chlorine and iron. With
the discovery that the corrosion product contained zinc, a new search was performed, and a
good match was found with the unusual mineral namuwite — which can be considered as a zinc-
substituted brochantite, (Zn,Cu) 4 S0 4 (OH) 6 -4H 2 0 —that was previously unreported as a cor
rosion product of art objects. Namuwite is a hexagonal mineral, a pearly sea-green when pure,
C O P P E R S U L F I D E S
233