Page 286 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 286
proteins, but they are extremely difficult to characterize. The most important group of organic
copper salts is the verdigris ensemble. These compounds are the result of many attempts at syn
thesizing copper greens that date from the early centuries B.C.E. through the late medieval
period and — i f copper phthalocyanins are included in this group—up to the modern era. From
the eighteenth century onward, however, chemists began to produce synthetic compounds that
gradually displaced the traditional pigments. The salts prepared by ancient and historic recipes
often have subtle differences in their composition that can make positive identification difficult.
T H E C O P P E R F O R M A T E S
Since formic acid and formaldehyde can be omnipresent pollutants in museum display and stor
age environments, it is surprising how little mention the copper formates have received in the
conservation literature to date. Evidence for the existence of copper formates as a patina com
ponent, in small concentrations, has come from the work of Graedel, McCrory-Joy, and Franey
(i986) and from corrosion studies of the Statue of Liberty (see CHAPTER 5). In the corrosion of
copper by wood, plywood, and laminates, there is a strong possibility that formate salts will be
identified, although there are currently no entries for the basic copper formates in the ICDD files.
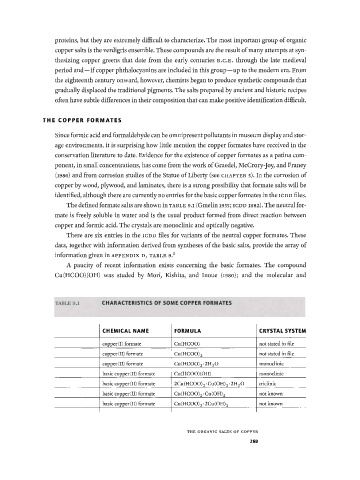
The defined formate salts are shown in TABLE 9.1 (Gmelin 1955; ICDD 1982). The neutral for
mate is freely soluble in water and is the usual product formed from direct reaction between
copper and formic acid. The crystals are monoclinic and optically negative.
There are six entries in the ICDD files for variants of the neutral copper formates. These
data, together with information derived from syntheses of the basic salts, provide the array of
information given in APPENDIX D, TABLE 9. 2
A paucity of recent information exists concerning the basic formates. The compound
Cu(HCOO)(OH) was studed by Mori, Kishita, and Inoue (i98o); and the molecular and
TABLE 9.1 C H A R A C T E R I S T I C S O F S O M E C O P P E R F O R M A T E S
C H E M I C A L N A M E F O R M U L A C R Y S T A L S Y S T E M
copper (I) formate Cu(HCOO) not stated in file
copper (II) formate Cu(HCOO) 2 not stated in file
copper (II) formate Cu(HC00) 2 -2H 2 0 monoclinic
basic copper (II) formate Cu(HCOO)(OH) monoclinic
basic copper (II) formate 2Cu (HCOO) 2 · Cu (OH) 2 · 2H 2 O triclinic
basic copper (II) formate Cu(HCOO) 2-Cu(OH) 2 not known
basic copper (II) formate Cu(HCOO) 2-2Cu(OH) 2 not known
T H E ORGANI C SALT S O F C O P P E R
269