Page 288 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 288
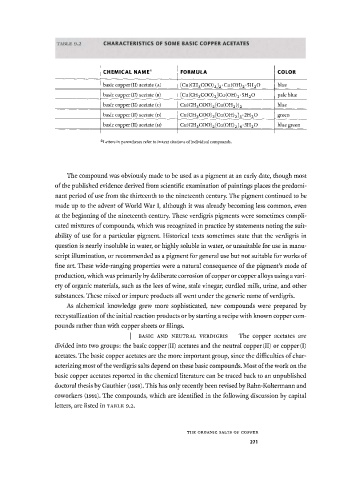
TABLE 9.2 CHARACTERISTICS OF SOME BASIC COPPER ACETATES
CHEMICAL NAME FORMULA COLOR
basic copper (II) acetate (A) [Cu (CH 3 COO) 2 ] 2 · Cu (OH) 2 · 5H 2 O blue
basic copper (11) acetate (B) [Cu(CH 3 COO) 2 ]Cu(OH) 2 -5H 2 0 pale blue
basic copper (11) acetate (c) Cu(CH 3 COO) 2 [Cu(OH 2 )] 2 blue
basic copper (II) acetate (D) Cu (CH 3 COO) 2 [Cu(OH) 2 ] 3 · 2H 2 O green
basic copper (II) acetate (H) Cu (CH 3 COO) 2 [Cu(OH) 2 ] 4 · 3H 2 O blue green
Letters in parentheses refer to in-text citations of individual compounds.
The compound was obviously made to be used as a pigment at an early date, though most
of the published evidence derived from scientific examination of paintings places the predomi
nant period of use from the thirteenth to the nineteenth century. The pigment continued to be
I
made up to the advent of World War , although it was already becoming less common, even
at the beginning of the nineteenth century. These verdigris pigments were sometimes compli
cated mixtures of compounds, which was recognized in practice by statements noting the suit
ability of use for a particular pigment. Historical texts sometimes state that the verdigris in
question is nearly insoluble in water, or highly soluble in water, or unsuitable for use in manu
script illumination, or recommended as a pigment for general use but not suitable for works of
fine art. These wide-ranging properties were a natural consequence of the pigment's mode of
production, which was primarily by deliberate corrosion of copper or copper alloys using a vari
ety of organic materials, such as the lees of wine, stale vinegar, curdled milk, urine, and other
substances. These mixed or impure products all went under the generic name of verdigris.
As alchemical knowledge grew more sophisticated, new compounds were prepared by
recrystallization of the initial reaction products or by starting a recipe with known copper com
pounds rather than with copper sheets or filings.
I BASIC AND NEUTRAL VERDIGRIS The copper acetates are
divided into two groups: the basic copper (II) acetates and the neutral copper (II) or copper (I)
acetates. The basic copper acetates are the more important group, since the difficulties of char
acterizing most of the verdigris salts depend on these basic compounds. Most of the work on the
basic copper acetates reported in the chemical literature can be traced back to an unpublished
doctoral thesis by Gauthier (i958). This has only recently been revised by Rahn-Koltermann and
coworkers (i99i). The compounds, which are identified in the following discussion by capital
letters, are listed in TABLE 9.2.
T H E O R G A N I C S A L T S O F C O P P E R
271