Page 395 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 395
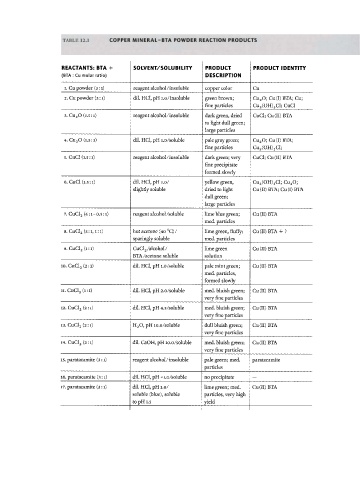
TABLE 12.1 C O P P E R M I N E R A L - B T A P O W D E R R E A C T I O N P R O D U C T S
R E A C T A N T S : BT A + S O L V E N T / S O L U B I L I T Y P R O D U C T P R O D U C T I D E N T I T Y
(BTA : Cu molar ratio) D E S C R I P T I O N
l. Cu powder (3:1) reagent alcohol/insoluble copper color Cu
2. Cu powder (3:1) dil. HCl, pH 1.0/insoluble green brown; Cu 2 0; Cu(I) BTA; Cu;
fine particles Cu 2 (OH) 3 Cl; CuCl
3. Cu 2 0 (1.5:1) reagent alcohol/insoluble dark green, dried CuCl; Cu(II) BTA
to light dull green;
large particles
4. Cu 2 0 (1.5:1) dil. HCl, pH í.o/soluble pale gray green; Cu 2 0; Cu(I) BTA;
fine particles Cu 2 (OH) 3 Cl;
5. CuCl (1.5:1) reagent alcohol/insoluble dark green; very CuCl; Cu(II) BTA
fine precipitate
formed slowly
6. CuCl (1.5:1) dil. HCl, pH 1.0/ yellow green, Cu 2 (OH) 3 Cl; Cu 2 0;
slightly soluble dried to light Cu(II)BTA; Cu(I)BTA
dull green;
large particles
( 4 : 1 - 0 . 5 : 1 ) reagent alcohol/soluble lime blue green; Cu(II) BTA
7. CuCl 2
med. particles
( 3 : 1 , 1 : 1 ) hot acetone (40 °C) / lime green, fluffy; Cu(II) BTA + ?
8. CuCl 2
sparingly soluble med. particles
(1:1) CuCl 2/alcohol/ lime green Cu(II) BTA
9. CuCl 2
ΒΤΑ /acetone soluble solution
10. CuCl 2 2:1) dil. HCl, pH 1.0/soluble pale mint green; Cu(II) BTA
(
med. particles,
formed slowly
(1:1)
11. CuCl 2 dil. HCl, pH 2.0/soluble med. bluish green; Cu(II) BTA
very fine particles
12. CuCl 2 2:1) dil. HCl, pH 4.5/soluble med. bluish green; Cu(II) BTA
(
very fine particles
13. CuCl 2 2:1) H 2 0, pH 10.0/soluble dull bluish green; Cu(II) BTA
(
very fine particles
(
14. CuCl 2 2:1) dil. CaOH, pH 10.0/soluble med. bluish green; Cu(II) BTA
very fine particles
15. paratacamite (3:1) reagent alcohol/insoluble pale green; med. paratacamite
particles
16. paratacamite (3:1) dil. HCl, pH <i.o/soluble no precipitate —
17. paratacamite (3:1) dil. HCl, pH 1.0/ lime green; med. Cu(II) BTA
soluble (blue), soluble particles, very high
to pH 1.5 yield