Page 396 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 396
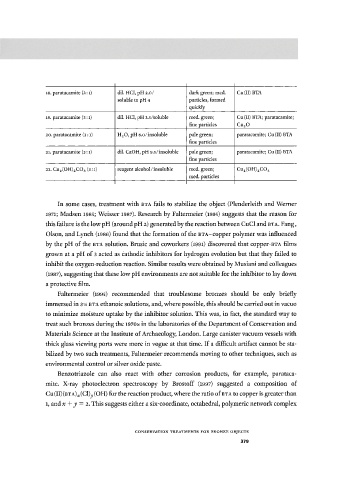
18. paratacamite (3:1) dil. HCl, pH 2.0 / dark green; med. Cu(II) BTA
soluble to pH 4 particles, formed
quickly
19. paratacamite (3:1) dil. HCl, pH 3.5/soluble med. green; Cu(II) BTA; paratacamite;
fine particles Cu 2 0
20. paratacamite (3:1) H 2 0, pH 6.0/insoluble pale green; paratacamite; Cu(II) BTA
fine particles
21. paratacamite (3:1) dil. CaOH, pH 9.5/insoluble pale green; paratacamite; Cu(II) BTA
fine particles
22. Cu 2 (OH) 2 C0 3 (3:i) reagent alcohol/insoluble med. green; Cu 2 (OH) 2 C0 3
med. particles
In some cases, treatment with B TA fails to stabilize the object (Plenderleith and Werner
i97i; Madsen 1985; Weisser 1987). Research by Faltermeier (1995) suggests that the reason for
this failure is the low pH (around pH 2) generated by the reaction between CuCl and BTA. Fang,
Olson, and Lynch (i986) found that the formation of the BTA- copper polymer was influenced
by the pH of the BTA solution. Brusic and coworkers (1991) discovered that copper-ΒΤΑ films
grown at a pH of 3 acted as cathodic inhibitors for hydrogen evolution but that they failed to
inhibit the oxygen-reduction reaction. Similar results were obtained by Musiani and colleagues
(1987), suggesting that these low pH environments are not suitable for the inhibitor to lay down
a protective film.
Faltermeier (1995) recommended that troublesome bronzes should be only briefly
immersed in 3% BTA ethanoic solutions, and, where possible, this should be carried out in vacuo
to minimize moisture uptake by the inhibitor solution. This was, in fact, the standard way to
treat such bronzes during the 1970s in the laboratories of the Department of Conservation and
Materials Science at the Institute of Archaeology, London. Large canister vacuum vessels with
I
thick glass viewing ports were more in vogue at that time. f a difficult artifact cannot be sta
bilized by two such treatments, Faltermeier recommends moving to other techniques, such as
environmental control or silver oxide paste.
Benzotriazole can also react with other corrosion products, for example, parataca-
mite. X-ray photoelectron spectroscopy by Brostoff (1997) suggested a composition of
CU(II)(BTA) A .(C1)J, (OH) for the reaction product, where the ratio of BTA to copper is greater than
1, and χ + y — 2. This suggests either a six-coordinate, octahedral, polymeric network complex
C O N S E R V A T I O N T R E A T M E N T S F O R B R O N Z E O B J E C T S
379