Page 4 - AAS & AES & FES 01082016_Neat
P. 4
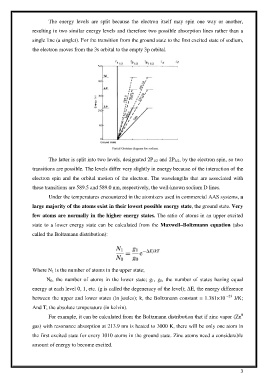
The energy levels are split because the electron itself may spin one way or another,
resulting in two similar energy levels and therefore two possible absorption lines rather than a
single line (a singlet). For the transition from the ground state to the first excited state of sodium,
the electron moves from the 3s orbital to the empty 3p orbital.
The latter is split into two levels, designated 2P 1/2 and 2P 3/2, by the electron spin, so two
transitions are possible. The levels differ very slightly in energy because of the interaction of the
electron spin and the orbital motion of the electron. The wavelengths that are associated with
these transitions are 589.5 and 589.0 nm, respectively, the well-known sodium D lines.
Under the temperatures encountered in the atomizers used in commercial AAS systems, a
large majority of the atoms exist in their lowest possible energy state, the ground state. Very
few atoms are normally in the higher energy states. The ratio of atoms in an upper excited
state to a lower energy state can be calculated from the Maxwell–Boltzmann equation (also
called the Boltzmann distribution):
Where N 1 is the number of atoms in the upper state;
N 0, the number of atoms in the lower state; g 1, g 0, the number of states having equal
energy at each level 0, 1, etc. (g is called the degeneracy of the level); ∆E, the energy difference
between the upper and lower states (in joules); k, the Boltzmann constant = 1.381×10 −23 J/K;
And T, the absolute temperature (in kelvin).
For example, it can be calculated from the Boltzmann distribution that if zinc vapor (Zn 0
gas) with resonance absorption at 213.9 nm is heated to 3000 K, there will be only one atom in
the first excited state for every 1010 atoms in the ground state. Zinc atoms need a considerable
amount of energy to become excited.
3