Page 17 - Demo
P. 17
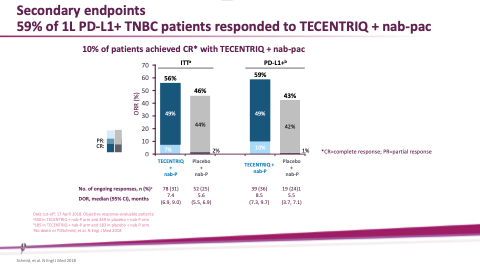
Secondary endpoints
59% of 1L PD-L1+ TNBC patients responded to TECENTRIQ + nab-pac
10% of patients achieved CR* with TECENTRIQ + nab-pac
70 60 50 40 30 20 10
0
ITT
56%
46%
PD-L1+ 59%
43%
ab
49%
49%
44%
49% 49%
42%
10%
7%
No. of ongoing responses, n (%)c DOR, median (95% CI), months
Data cut-off: 17 April 2018. Objective response-evaluable patients: a450 in TECENTRIQ + nab-P arm and 449 in placebo + nab-P arm b185 in TECENTRIQ + nab-P arm and 183 in placebo + nab-P arm. cNo death or PDSchmid, et al. N Engl J Med 2018
78 (31) 7.4 (6.9, 9.0)
52 (25) 5.6 (5.5, 6.9)
39 (36) 8.5 (7.3, 9.7)
19 (24)1 5.5 (3.7, 7.1)
PR: CR:
2%
1%
*CR=complete response; PR=partial response
ITT A-
ITT P-
PD-L1+
PD-L1+
TECENTRIQ
Placebo
Placebo
TECENTRIQ + +++
nabPx
nabPx
A-nabb-PPx P-nabPx nab-P
nab-P
nab-P
Schmid, et al. N Engl J Med 2018
ORR (%)