Page 10 - pfizervax
P. 10
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
Objectives, Estimands, and Endpoints
For Phase 1
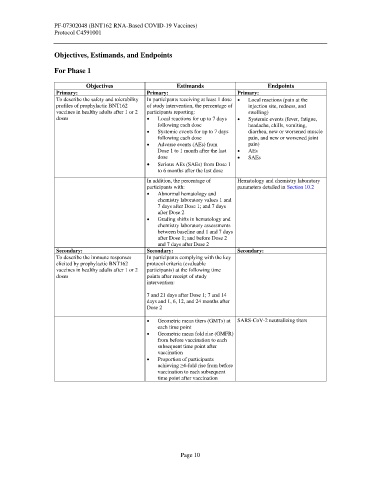
Objectives Estimands Endpoints
Primary: Primary: Primary:
To describe the safety and tolerability In participants receiving at least 1 dose • Local reactions (pain at the
profiles of prophylactic BNT162 of study intervention, the percentage of injection site, redness, and
vaccines in healthy adults after 1 or 2 participants reporting: swelling)
doses • Local reactions for up to 7 days • Systemic events (fever, fatigue,
following each dose headache, chills, vomiting,
• Systemic events for up to 7 days diarrhea, new or worsened muscle
following each dose pain, and new or worsened joint
• Adverse events (AEs) from pain)
Dose 1 to 1 month after the last • AEs
dose • SAEs
• Serious AEs (SAEs) from Dose 1
to 6 months after the last dose
In addition, the percentage of Hematology and chemistry laboratory
participants with: parameters detailed in Section 10.2
• Abnormal hematology and
chemistry laboratory values 1 and
7 days after Dose 1; and 7 days
after Dose 2
• Grading shifts in hematology and
chemistry laboratory assessments
between baseline and 1 and 7 days
after Dose 1; and before Dose 2
and 7 days after Dose 2
Secondary: Secondary: Secondary:
To describe the immune responses In participants complying with the key
elicited by prophylactic BNT162 protocol criteria (evaluable
vaccines in healthy adults after 1 or 2 participants) at the following time
doses points after receipt of study
intervention:
7 and 21 days after Dose 1; 7 and 14
days and 1, 6, 12, and 24 months after
Dose 2
• Geometric mean titers (GMTs) at SARS-CoV-2 neutralizing titers
each time point
• Geometric mean fold rise (GMFR)
from before vaccination to each
subsequent time point after
vaccination
• Proportion of participants
achieving ≥4-fold rise from before
vaccination to each subsequent
time point after vaccination
Page 10