Page 13 - pfizervax
P. 13
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
a
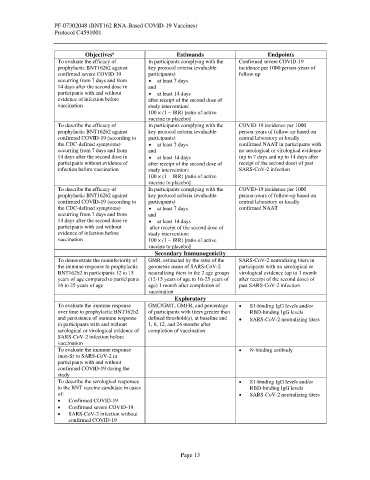
Objectives Estimands Endpoints
To evaluate the efficacy of In participants complying with the Confirmed severe COVID-19
prophylactic BNT162b2 against key protocol criteria (evaluable incidence per 1000 person-years of
confirmed severe COVID-19 participants) follow-up
occurring from 7 days and from • at least 7 days
14 days after the second dose in and
participants with and without • at least 14 days
evidence of infection before after receipt of the second dose of
vaccination study intervention:
100 × (1 – IRR) [ratio of active
vaccine to placebo]
To describe the efficacy of In participants complying with the COVID-19 incidence per 1000
prophylactic BNT162b2 against key protocol criteria (evaluable person-years of follow-up based on
confirmed COVID-19 (according to participants) central laboratory or locally
the CDC-defined symptoms) • at least 7 days confirmed NAAT in participants with
occurring from 7 days and from and no serological or virological evidence
14 days after the second dose in • at least 14 days (up to 7 days and up to 14 days after
participants without evidence of after receipt of the second dose of receipt of the second dose) of past
infection before vaccination study intervention: SARS-CoV-2 infection
100 × (1 – IRR) [ratio of active
vaccine to placebo]
To describe the efficacy of In participants complying with the COVID-19 incidence per 1000
prophylactic BNT162b2 against key protocol criteria (evaluable person-years of follow-up based on
confirmed COVID-19 (according to participants) central laboratory or locally
the CDC-defined symptoms) • at least 7 days confirmed NAAT
occurring from 7 days and from and
14 days after the second dose in • at least 14 days
participants with and without after receipt of the second dose of
evidence of infection before study intervention:
vaccination 100 × (1 – IRR) [ratio of active
vaccine to placebo]
Secondary Immunogenicity
To demonstrate the noninferiority of GMR, estimated by the ratio of the SARS-CoV-2 neutralizing titers in
the immune response to prophylactic geometric mean of SARS-CoV-2 participants with no serological or
BNT162b2 in participants 12 to 15 neutralizing titers in the 2 age groups virological evidence (up to 1 month
years of age compared to participants (12-15 years of age to 16-25 years of after receipt of the second dose) of
16 to 25 years of age age) 1 month after completion of past SARS-CoV-2 infection
vaccination
Exploratory
To evaluate the immune response GMC/GMT, GMFR, and percentage • S1-binding IgG levels and/or
over time to prophylactic BNT162b2 of participants with titers greater than RBD-binding IgG levels
and persistence of immune response defined threshold(s), at baseline and • SARS-CoV-2 neutralizing titers
in participants with and without 1, 6, 12, and 24 months after
serological or virological evidence of completion of vaccination
SARS-CoV-2 infection before
vaccination
To evaluate the immune response • N-binding antibody
(non-S) to SARS-CoV-2 in
participants with and without
confirmed COVID-19 during the
study
To describe the serological responses • S1-binding IgG levels and/or
to the BNT vaccine candidate in cases RBD-binding IgG levels
of: • SARS-CoV-2 neutralizing titers
• Confirmed COVID-19
• Confirmed severe COVID-19
• SARS-CoV-2 infection without
confirmed COVID-19
Page 13