Page 117 - pfizervax
P. 117
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
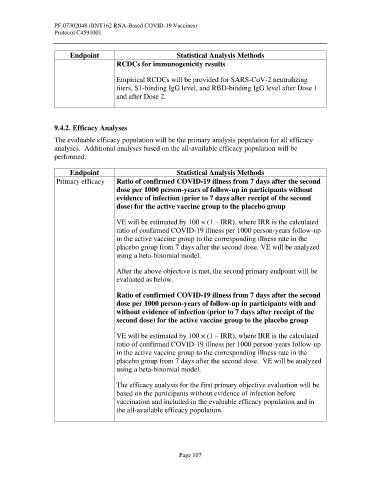
Endpoint Statistical Analysis Methods
RCDCs for immunogenicity results
Empirical RCDCs will be provided for SARS-CoV-2 neutralizing
titers, S1-binding IgG level, and RBD-binding IgG level after Dose 1
and after Dose 2.
9.4.2. Efficacy Analyses
The evaluable efficacy population will be the primary analysis population for all efficacy
analyses. Additional analyses based on the all-available efficacy population will be
performed.
Endpoint Statistical Analysis Methods
Primary efficacy Ratio of confirmed COVID-19 illness from 7 days after the second
dose per 1000 person-years of follow-up in participants without
evidence of infection (prior to 7 days after receipt of the second
dose) for the active vaccine group to the placebo group
VE will be estimated by 100 × (1 – IRR), where IRR is the calculated
ratio of confirmed COVID-19 illness per 1000 person-years follow-up
in the active vaccine group to the corresponding illness rate in the
placebo group from 7 days after the second dose. VE will be analyzed
using a beta-binomial model.
After the above objective is met, the second primary endpoint will be
evaluated as below.
Ratio of confirmed COVID-19 illness from 7 days after the second
dose per 1000 person-years of follow-up in participants with and
without evidence of infection (prior to 7 days after receipt of the
second dose) for the active vaccine group to the placebo group
VE will be estimated by 100 × (1 – IRR), where IRR is the calculated
ratio of confirmed COVID-19 illness per 1000 person-years follow-up
in the active vaccine group to the corresponding illness rate in the
placebo group from 7 days after the second dose. VE will be analyzed
using a beta-binomial model.
The efficacy analysis for the first primary objective evaluation will be
based on the participants without evidence of infection before
vaccination and included in the evaluable efficacy population and in
the all-available efficacy population.
Page 107