Page 122 - pfizervax
P. 122
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
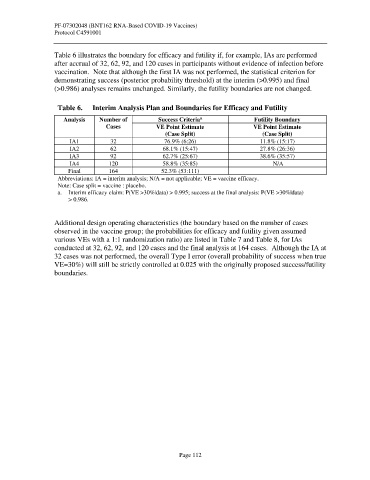
Table 6 illustrates the boundary for efficacy and futility if, for example, IAs are performed
after accrual of 32, 62, 92, and 120 cases in participants without evidence of infection before
vaccination. Note that although the first IA was not performed, the statistical criterion for
demonstrating success (posterior probability threshold) at the interim (>0.995) and final
(>0.986) analyses remains unchanged. Similarly, the futility boundaries are not changed.
Table 6. Interim Analysis Plan and Boundaries for Efficacy and Futility
a
Analysis Number of Success Criteria Futility Boundary
Cases VE Point Estimate VE Point Estimate
(Case Split) (Case Split)
IA1 32 76.9% (6:26) 11.8% (15:17)
IA2 62 68.1% (15:47) 27.8% (26:36)
IA3 92 62.7% (25:67) 38.6% (35:57)
IA4 120 58.8% (35:85) N/A
Final 164 52.3% (53:111)
Abbreviations: IA = interim analysis; N/A = not applicable; VE = vaccine efficacy.
Note: Case split = vaccine : placebo.
a. Interim efficacy claim: P(VE >30%|data) > 0.995; success at the final analysis: P(VE >30%|data)
> 0.986.
Additional design operating characteristics (the boundary based on the number of cases
observed in the vaccine group; the probabilities for efficacy and futility given assumed
various VEs with a 1:1 randomization ratio) are listed in Table 7 and Table 8, for IAs
conducted at 32, 62, 92, and 120 cases and the final analysis at 164 cases. Although the IA at
32 cases was not performed, the overall Type I error (overall probability of success when true
VE=30%) will still be strictly controlled at 0.025 with the originally proposed success/futility
boundaries.
Page 112