Page 19 - pfizervax
P. 19
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
1.3. Schedule of Activities
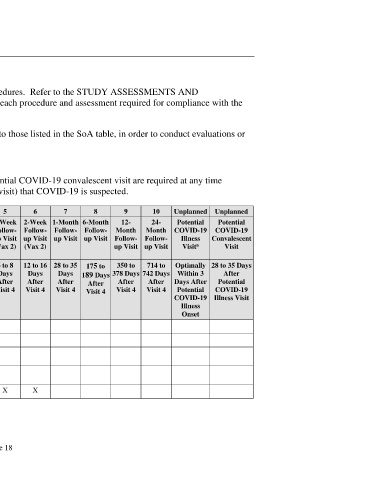
The SoA table provides an overview of the protocol visits and procedures. Refer to the STUDY ASSESSMENTS AND
PROCEDURES section of the protocol for detailed information on each procedure and assessment required for compliance with the
protocol.
The investigator may schedule visits (unplanned visits) in addition to those listed in the SoA table, in order to conduct evaluations or
assessments required to protect the well-being of the participant.
1.3.1. Phase 1
An unplanned potential COVID-19 illness visit and unplanned potential COVID-19 convalescent visit are required at any time
between Visit 1 (Vaccination 1) and Visit 10 (24-month follow-up visit) that COVID-19 is suspected.
Visit Number Screening 1 2 3 4 5 6 7 8 9 10 Unplanned Unplanned
Visit Description Screening Vax 1 Next- 1-Week Vax 2 1-Week 2-Week 1-Month 6-Month 12- 24- Potential Potential
Day Follow- Follow- Follow- Follow- Follow- Month Month COVID-19 COVID-19
Follow- up Visit up Visit up Visit up Visit up Visit Follow- Follow- Illness Convalescent
a
up Visit (Vax 1) (Vax 2) (Vax 2) up Visit up Visit Visit Visit
(Vax 1)
Visit Window (Days) 0 to 28 Day 1 1 to 3 6 to 8 19 to 23 6 to 8 12 to 16 28 to 35 175 to 350 to 714 to Optimally 28 to 35 Days
Days Days Days Days Days Days Days 189 Days 378 Days 742 Days Within 3 After
Before After After After After After After After After After Days After Potential
Visit 1 Visit 1 Visit 1 Visit 1 Visit 4 Visit 4 Visit 4 Visit 4 Visit 4 Visit 4 Potential COVID-19
COVID-19 Illness Visit
Illness
Onset
Obtain informed consent X
Assign participant number X
Obtain demography and X
medical history data
Obtain details of medications X
currently taken
Perform physical examination X X X X X X X
Page 18