Page 21 - pfizervax
P. 21
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
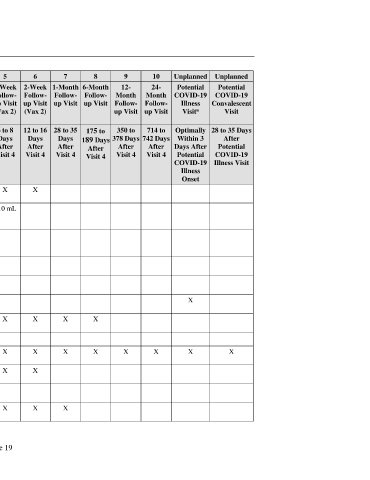
Visit Number Screening 1 2 3 4 5 6 7 8 9 10 Unplanned Unplanned
Visit Description Screening Vax 1 Next- 1-Week Vax 2 1-Week 2-Week 1-Month 6-Month 12- 24- Potential Potential
Day Follow- Follow- Follow- Follow- Follow- Month Month COVID-19 COVID-19
Follow- up Visit up Visit up Visit up Visit up Visit Follow- Follow- Illness Convalescent
up Visit (Vax 1) (Vax 2) (Vax 2) up Visit up Visit Visit Visit
a
(Vax 1)
Visit Window (Days) 0 to 28 Day 1 1 to 3 6 to 8 19 to 23 6 to 8 12 to 16 28 to 35 175 to 350 to 714 to Optimally 28 to 35 Days
Days Days Days Days Days Days Days 189 Days 378 Days 742 Days Within 3 After
Before After After After After After After After After After Days After Potential
Visit 1 Visit 1 Visit 1 Visit 1 Visit 4 Visit 4 Visit 4 Visit 4 Visit 4 Visit 4 Potential COVID-19
COVID-19 Illness Visit
Illness
Onset
Measure vital signs X X X X X X X
(including body temperature)
Collect blood sample for ~10 mL ~10 mL ~10 mL ~10 mL ~10 mL
hematology and chemistry
b
laboratory tests
Collect screening blood ~10 mL
sample for HIV, HBsAg,
HBc Ab, and HCV Ab tests
Serological test for prior ~20 mL
COVID-19 infection
Perform urine pregnancy test X X X
(if appropriate)
Obtain nasal (midturbinate) X X X
c
swab(s)
Collect nonstudy vaccine X X X X X X X X X
information
Confirm eligibility X X X
Collect prohibited medication X X X X X X X X X X X
use
Review hematology and X X X X X
chemistry results
Review temporary delay X X
criteria
Confirm use of contraceptives X X X X X X X X
(if appropriate)
Page 19