Page 27 - pfizervax
P. 27
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
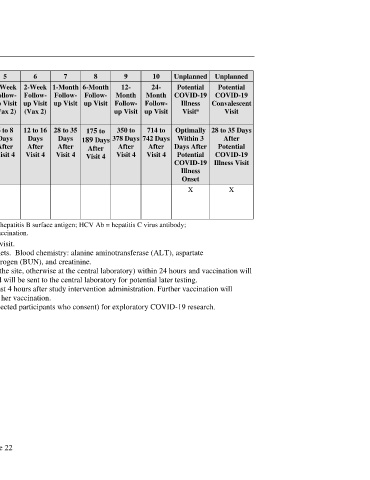
Visit Number Screening 1 2 3 4 5 6 7 8 9 10 Unplanned Unplanned
Visit Description Screening Vax 1 Next- 1-Week Vax 2 1-Week 2-Week 1-Month 6-Month 12- 24- Potential Potential
Day Follow- Follow- Follow- Follow- Follow- Month Month COVID-19 COVID-19
Follow- up Visit up Visit up Visit up Visit up Visit Follow- Follow- Illness Convalescent
a
up Visit (Vax 1) (Vax 2) (Vax 2) up Visit up Visit Visit Visit
(Vax 1)
Visit Window (Days) 0 to 28 Day 1 1 to 3 6 to 8 19 to 23 6 to 8 12 to 16 28 to 35 175 to 350 to 714 to Optimally 28 to 35 Days
Days Days Days Days Days Days Days 189 Days 378 Days 742 Days Within 3 After
Before After After After After After After After After After Days After Potential
Visit 1 Visit 1 Visit 1 Visit 1 Visit 4 Visit 4 Visit 4 Visit 4 Visit 4 Visit 4 Potential COVID-19
COVID-19 Illness Visit
Illness
Onset
Collection of X X
COVID-19–related clinical
and laboratory information
(including local diagnosis)
Abbreviations: e-diary = electronic diary; HBc Ab = hepatitis B core antibody; HBsAg = hepatitis B surface antigen; HCV Ab = hepatitis C virus antibody;
HIV = human immunodeficiency virus; NAAT = nucleic acid amplification test; vax = vaccination.
a. The COVID-19 illness visit may be conducted as an in-person or telehealth visit.
b. Hematology: hemoglobin, complete blood count with differential, and platelets. Blood chemistry: alanine aminotransferase (ALT), aspartate
aminotransferase (AST), alkaline phosphatase, total bilirubin, blood urea nitrogen (BUN), and creatinine.
c. Two swabs will be taken at Visits 1 and 4. One will be tested (if possible at the site, otherwise at the central laboratory) within 24 hours and vaccination will
only proceed if it is NAAT-negative for SARS-CoV-2 genomes. The second will be sent to the central laboratory for potential later testing.
d. The first 5 participants in in each group will be observed at the site for at least 4 hours after study intervention administration. Further vaccination will
commence no sooner than 24 hours after the fifth participant received his or her vaccination.
e. An optional blood draw of ~170 mL will be taken at 1 of the visits (from selected participants who consent) for exploratory COVID-19 research.
Page 22