Page 30 - pfizervax
P. 30
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
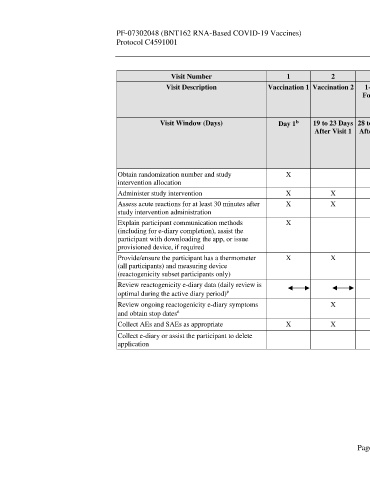
Visit Number 1 2 3 4 5 6 Unplanned Unplanned
Visit Description Vaccination 1 Vaccination 2 1-Month 6-Month 12-Month 24-Month Potential Potential
Follow-up Follow-up Follow-up Follow-up COVID-19 COVID-19
Visit Visit Visit Visit Illness Visit Convalescent
a
Visit
b
Visit Window (Days) Day 1 19 to 23 Days 28 to 35 Days 175 to 189 350 to 378 714 to 742 Optimally 28 to 35 Days
After Visit 1 After Visit 2 Days After Days After Days After Within 3 After
Visit 2 Visit 2 Visit 2 Days After Potential
Potential COVID-19
COVID-19 Illness Visit
Illness Onset
Obtain randomization number and study X
intervention allocation
Administer study intervention X X
Assess acute reactions for at least 30 minutes after X X
study intervention administration
Explain participant communication methods X
(including for e-diary completion), assist the
participant with downloading the app, or issue
provisioned device, if required
Provide/ensure the participant has a thermometer X X
(all participants) and measuring device
(reactogenicity subset participants only)
Review reactogenicity e-diary data (daily review is
optimal during the active diary period) e
Review ongoing reactogenicity e-diary symptoms X X
and obtain stop dates e
f
f
Collect AEs and SAEs as appropriate X X X X X X X X
f
f
Collect e-diary or assist the participant to delete X
application
Page 24