Page 25 - Laboratory manual for students FAR222 2019 20
P. 25
FAR 222 Dosage Form II Laboratory Manual
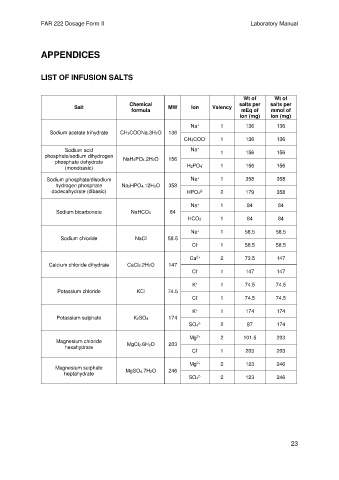
APPENDICES
LIST OF INFUSION SALTS
Wt of Wt of
Chemical salts per salts per
Salt MW Ion Valency
formula mEq of mmol of
ion (mg) ion (mg)
+
Na 1 136 136
Sodium acetate trihydrate CH3COONa.3H2O 136
CH3COO 1 136 136
-
+
Sodium acid Na 1 156 156
phosphate/sodium dihydrogen NaH2PO4.2H2O 156
phosphate dehydrate -
(monobasic) H2PO4 1 156 156
+
Sodium phosphate/disodium Na 1 358 358
hydrogen phosphate Na2HPO4.12H2O 358
dodecahydrate (dibasic) HPO4 2 179 358
2-
+
Na 1 84 84
Sodium bicarbonate NaHCO3 84
-
HCO3 1 84 84
Na 1 58.5 58.5
+
Sodium chloride NaCl 58.5
Cl - 1 58.5 58.5
2+
Ca 2 73.5 147
Calcium chloride dihydrate CaCl2.2H2O 147
Cl - 1 147 147
K 1 74.5 74.5
+
Potassium chloride KCl 74.5
Cl - 1 74.5 74.5
+
K 1 174 174
Potassium sulphate K2SO4 174
SO4 2 87 174
2-
2+
Mg 2 101.5 203
Magnesium chloride MgCl2.6H2O 203
hexahydrate
Cl - 1 203 203
2+
Mg 2 123 246
Magnesium sulphate MgSO4.7H2O 246
heptahydrate
2-
SO4 2 123 246
23