Page 10 - CBAC Newsletter 2013
P. 10
Conclusions
The proposed link between ERS and K ATP genetic mutations has recently gained significant traction, particularly with
reports of decreased ATP sensitivity in mutant channels (Barajas-Martinez, Hu et al. 2012). However, many questions
still remain regarding the physiological function and molecular constituents of K in human myocytes (Nichols, Singh
ATP
et al. 2013). To make further progress, a quantitative understanding of the distribution of human K forming sub-
ATP
units, the ATP-sensitivity of the channels affected, potential consequences of other modifiers such as PIP2 and the
heterogeneous consequences of channel activation are required. As demonstrated by the species variation between
mouse and canine, small animal models may not serve as an appropriate proxy for studies in native human myocytes.
Furthermore, even if SUR1 and Kir6.1 expression is less than Kir6.2 and SUR2, differential sensitivity to intracellular
modification and the ability of even a small number of active channels to dramatically reduce APD may allow for a
significant role for these subunits.
As the primary link between cardiac myocyte metabolism and excitability, K channels offer a gateway to linking the
ATP
rapidly emerging field of metabolomics to cardiac electrophysiology modeling. Certainly, how intracellular metabolitic
factors regulate K and other major AP forming channels will need to be parameterized, in addition to theoretical
ATP
frameworks that link the two approaches. However, much stands to be gained from understanding how changes in
metabolites affect cardiac rhythm, in particular the acute and chronic consequences of ischemia that affect many
patients worldwide.
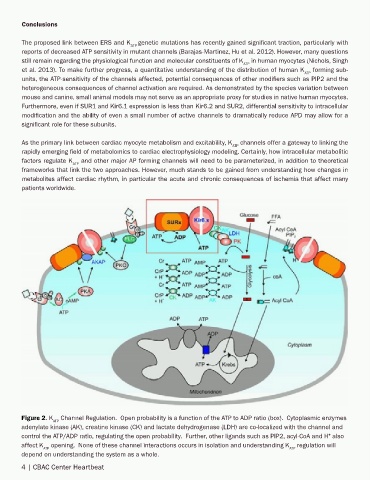
Figure 2. K Channel Regulation. Open probability is a function of the ATP to ADP ratio (box). Cytoplasmic enzymes
ATP
adenylate kinase (AK), creatine kinase (CK) and lactate dehydrogenase (LDH) are co-localized with the channel and
control the ATP/ADP ratio, regulating the open probability. Further, other ligands such as PIP2, acyl-CoA and H also
+
affect K opening. None of these channel interactions occurs in isolation and understanding K regulation will
ATP ATP
depend on understanding the system as a whole.
4 | CBAC Center Heartbeat