Page 923 - Adams and Stashak's Lameness in Horses, 7th Edition
P. 923
Principles of Therapy for Lameness 889
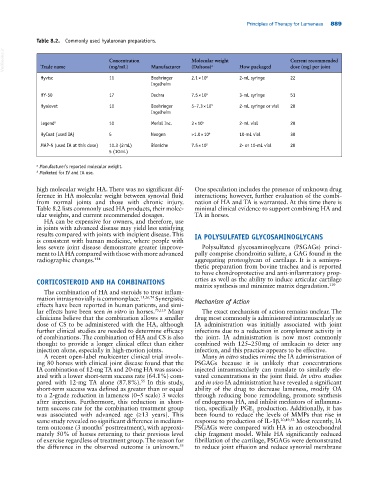
Table 8.2. Commonly used hyaluronan preparations.
VetBooks.ir Trade name Concentration Manufacturer Molecular weight How packaged Current recommended
dose (mg) per joint
(mg/mL)
(Daltons)
a
Hyvisc 11 Boehringer 2.1 × 10 6 2‐mL syringe 22
Ingelheim
HY‐50 17 Dechra 7.5 × 10 5 3‐mL syringe 51
Hyalovet 10 Boehringer 5–7.3 × 10 5 2‐mL syringe or vial 20
Ingelheim
Legend b 10 Merial Inc. 3 × 10 5 2‐mL vial 20
HyCoat (used IA) 5 Neogen >1.0 × 10 6 10‐mL vial 30
MAP‐5 (used IA at this dose) 10.3 (2 mL) Bioniche 7.5 × 10 5 2‐ or 10‐mL vial 20
5 (10 mL)
a Manufacturer’s reported molecular weight.
b Marketed for IV and IA use.
high molecular weight HA. There was no significant dif- One speculation includes the presence of unknown drug
ference in HA molecular weight between synovial fluid interactions; however, further evaluation of the combi-
from normal joints and those with chronic injury. nation of HA and TA is warranted. At this time there is
Table 8.2 lists commonly used HA products, their molec- minimal clinical evidence to support combining HA and
ular weights, and current recommended dosages. TA in horses.
HA can be expensive for owners, and therefore, use
in joints with advanced disease may yield less satisfying
results compared with joints with incipient disease. This IA POLYSULFATED GLYCOSAMINOGLYCANS
is consistent with human medicine, where people with
less severe joint disease demonstrate greater improve- Polysulfated glycosaminoglycans (PSGAGs) princi-
ment to IA HA compared with those with more advanced pally comprise chondroitin sulfate, a GAG found in the
radiographic changes. 114 aggregating proteoglycan of cartilage. It is a semisyn-
thetic preparation from bovine trachea and is reported
to have chondroprotective and anti‐inflammatory prop-
CORTICOSTEROID AND HA COMBINATIONS erties as well as the ability to induce articular cartilage
matrix synthesis and minimize matrix degradation.
110
The combination of HA and steroids to treat inflam-
mation intrasynovially is commonplace. 15,26,74 Synergistic Mechanism of Action
effects have been reported in human patients, and simi-
lar effects have been seen in vitro in horses. 75,119 Many The exact mechanism of action remains unclear. The
clinicians believe that the combination allows a smaller drug most commonly is administered intramuscularly as
dose of CS to be administered with the HA, although IA administration was initially associated with joint
further clinical studies are needed to determine efficacy infections due to a reduction in complement activity in
of combinations. The combination of HA and CS is also the joint. IA administration is now most commonly
thought to provide a longer clinical effect than either combined with 125–250 mg of amikacin to deter any
injection alone, especially in high‐motion joints. infection, and this practice appears to be effective.
A recent open‐label multicenter clinical trial involv- Many in vitro studies mimic the IA administration of
ing 80 horses with clinical joint disease found that the PSGAGs because it is unlikely that concentrations
IA combination of 12‐mg TA and 20‐mg HA was associ- injected intramuscularly can translate to similarly ele-
ated with a lower short‐term success rate (64.1%) com- vated concentrations in the joint fluid. In vitro studies
pared with 12‐mg TA alone (87.8%). In this study, and in vivo IA administration have revealed a significant
55
short‐term success was defined as greater than or equal ability of the drug to decrease lameness, modify OA
to a 2‐grade reduction in lameness (0–5 scale) 3 weeks through reducing bone remodeling, promote synthesis
after injection. Furthermore, this reduction in short‐ of endogenous HA, and inhibit mediators of inflamma-
term success rate for the combination treatment group tion, specifically PGE production. Additionally, it has
2
was associated with advanced age (≥13 years). This been found to reduce the levels of MMPs that rise in
same study revealed no significant difference in medium‐ response to production of IL‐1β. 20,49,52 Most recently, IA
term outcome (3 months’ posttreatment), with approxi- PSGAGs were compared with HA in an osteochondral
mately 50% of horses returning to their previous level chip fragment model. While HA significantly reduced
of exercise regardless of treatment group. The reason for fibrillation of the cartilage, PSGAGs were demonstrated
the difference in the observed outcome is unknown. to reduce joint effusion and reduce synovial membrane
55