Page 86 - Clinical Small Animal Internal Medicine
P. 86
54 Section 2 Endocrine Disease
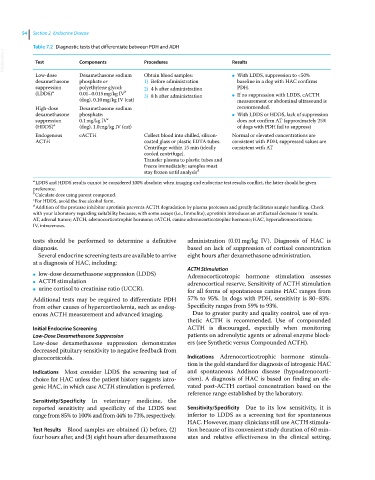
Table 7.2 Diagnostic tests that differentiate between PDH and ADH
VetBooks.ir Test Components Procedures Results
Low‐dose Dexamethasone sodium Obtain blood samples: ● With LDDS, suppression to <50%
dexamethasone phosphate or 1) Before administration baseline in a dog with HAC confirms
suppression polyethylene glycol: 2) 4 h after administration PDH.
b
(LDDS) a 0.01–0.015 mg/kg IV 3) 8 h after administration ● If no suppression with LDDS, cACTH
(dog), 0.10 mg/kg IV (cat) measurement or abdominal ultrasound is
High‐dose Dexamethasone sodium recommended.
dexamethasone phosphate: ● With LDDS or HDDS, lack of suppression
suppression 0.1 mg/kg IV c does not confirm AT (approximately 25%
(HDDS) a (dog), 1.0 mg/kg IV (cat) of dogs with PDH fail to suppress)
Endogenous cACTH Collect blood into chilled, silicon‐ Normal or elevated concentrations are
ACTH coated glass or plastic EDTA tubes. consistent with PDH, suppressed values are
Centrifuge within 15 min (ideally consistent with AT
cooled centrifuge).
Transfer plasma to plastic tubes and
freeze immediately; samples must
stay frozen until analysis d
a LDDS and HDDS results cannot be considered 100% absolute; when imaging and endocrine test results conflict, the latter should be given
preference.
b Calculate dose using parent compound.
c For HDDS, avoid the free alcohol form.
d Addition of the protease inhibitor aprotinin prevents ACTH degradation by plasma proteases and greatly facilitates sample handling. Check
with your laboratory regarding suitability because, with some assays (i.e., Immulite), aprotinin introduces an artifactual decrease in results.
AT, adrenal tumor; ATCH, adrenocorticotrophic hormone; cATCH, canine adrenocorticotrophic hormone; HAC, hyperadrenocorticism;
IV, intravenous.
tests should be performed to determine a definitive administration (0.01 mg/kg IV). Diagnosis of HAC is
diagnosis. based on lack of suppression of cortisol concentration
Several endocrine screening tests are available to arrive eight hours after dexamethasone administration.
at a diagnosis of HAC, including:
ACTH Stimulation
low‐dose dexamethasone suppression (LDDS)
● Adrenocorticotropic hormone stimulation assesses
ACTH stimulation
● adrenocortical reserve. Sensitivity of ACTH stimulation
urine cortisol to creatinine ratio (UCCR).
● for all forms of spontaneous canine HAC ranges from
Additional tests may be required to differentiate PDH 57% to 95%. In dogs with PDH, sensitivity is 80–83%.
from other causes of hypercortisolemia, such as endog Specificity ranges from 59% to 93%.
enous ACTH measurement and advanced imaging. Due to greater purity and quality control, use of syn
thetic ACTH is recommended. Use of compounded
Initial Endocrine Screening ACTH is discouraged, especially when monitoring
Low‐Dose Dexamethasone Suppression patients on adrenolytic agents or adrenal enzyme block
Low‐dose dexamethasone suppression demonstrates ers (see Synthetic versus Compounded ACTH).
decreased pituitary sensitivity to negative feedback from
glucocorticoids. Indications Adrenocorticotrophic hormone stimula
tion is the gold standard for diagnosis of iatrogenic HAC
Indications Most consider LDDS the screening test of and spontaneous Addison disease (hypoadrenocorti
choice for HAC unless the patient history suggests iatro cism). A diagnosis of HAC is based on finding an ele
genic HAC, in which case ACTH stimulation is preferred. vated post‐ACTH cortisol concentration based on the
reference range established by the laboratory.
Sensitivity/Specificity In veterinary medicine, the
reported sensitivity and specificity of the LDDS test Sensitivity/Specificity Due to its low sensitivity, it is
range from 85% to 100% and from 44% to 73%, respectively. inferior to LDDS as a screening test for spontaneous
HAC. However, many clinicians still use ACTH stimula
Test Results Blood samples are obtained (1) before, (2) tion because of its convenient study duration of 60 min
four hours after, and (3) eight hours after dexamethasone utes and relative effectiveness in the clinical setting,