Page 213 - Clinical Small Animal Internal Medicine
P. 213
18 Pathophysiology of Heart Failure 181
Progression between these phases seems most dependent ● alterations in myocardial energetics. These alterations
VetBooks.ir on the magnitude and type of overload wherein acute pres- reduce the number of viable myocytes and/or decrease
the intrinsic contractility of individual myocytes,
sure overload triggers the earliest onset and fastest rate of
hypertrophy. It has been postulated that while hypertrophy
thereby impairing cardiac performance.
prevents acute cardiac insufficiency, the unbalanced The previously beneficial augmentation of preload and
“growth” at the level of the organ, tissue, cell, and intracel- afterload now only serves to promote development of
lular organelles ultimately becomes the cause of chronic congestion or amplify contractility‐afterload mismatch.
cardiac insufficiency of the hypertrophied heart.
This cycle of disease progression continues as venous
pressures continue to rise, cardiac output declines, con-
Phase 3 – Transition to Heart Failure (Figure 18.1) gestion develops, and clinical signs of heart failure begin
Hypertrophy may be unable to maintain cardiac output to emerge.
in the face of chronic and progressive cardiovascular dis-
ease and the previously beneficial short‐ and long‐term
compensatory mechanisms ultimately prove detrimen- Myocyte Loss
tal. The transition from compensated hypertrophy to the A reduction in the number of viable myocytes or dys-
exhaustion phase is mediated by molecular mechanisms function of the viable myocytes may account for a com-
that produce: ponent of reduced myocardial contractile function.
Cardiomyocyte death appears to occur via necrosis,
myocyte growth
● apoptosis, and autophagy although it is uncertain if all
reexpression of fetal myocyte phenotype with a reduc-
● three are distinctive events or a continuum of overlap-
tion in the expression of the adult phenotype
alteration in the expression and/or function of the ping processes. Exposure of norepinephrine to cultured
● mammalian cardiomyocytes produces a concentration‐
proteins involved in excitation‐contraction coupling
necrosis and apoptosis of cardiomyocytes dependent decrease in viability and pathophysiologic
● levels of AT II also promote myocytolysis. Therefore, it
changes within the extracellular matrix
●
seems that myocyte loss can occur via mechanisms
beyond ischemia and many of these mechanisms, includ-
Congestion ing catecholamines, AT II, reactive oxygen species, nitric
oxide, inflammatory cytokines and mechanical strain,
are increased in the failing myocardium.
3 Reexpression of Fetal and Neonatal Genes and Altered
Contractile Proteins
Heart failure seems to alter both quantitative and qualita-
Ventricular Performance 1 2 tive protein expression, which may impair cardiac contrac-
tility. Isoforms of contractile proteins that are present
rapid, have been identified in some animal models of hyper-
Increased Afterload during fetal and neonatal life, when protein synthesis is
trophy and myocardial failure. Pressure overload hypertro-
phy in rats produces a shift from the rapid myosin heavy
chain (MHC) isoform (V 1 ) to the slow MHC isoform (V 3 ),
enabling normal tension generation but at a lower energy
Low Output
cost by reaching the tension more slowly. However, in spe-
Preload cies with a predominant V 3 ventricular MHC isoform,
including dogs, cats, and humans, this change seems less
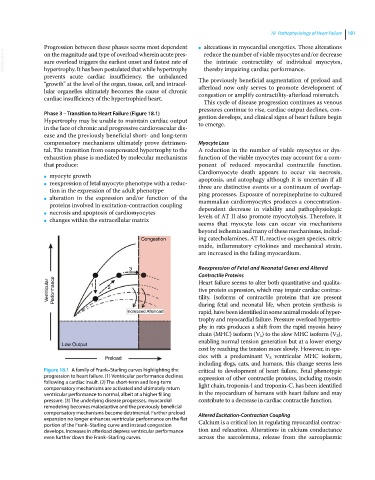
Figure 18.1 A family of Frank–Starling curves highlighting the critical to development of heart failure. Fetal phenotypic
progression to heart failure. (1) Ventricular performance declines expression of other contractile proteins, including myosin
following a cardiac insult. (2) The short‐term and long‐term light chain, troponin‐I and troponin‐C, has been identified
compensatory mechanisms are activated and ultimately return
ventricular performance to normal, albeit at a higher filling in the myocardium of humans with heart failure and may
pressure. (3) The underlying disease progresses, myocardial contribute to a decrease in cardiac contractile function.
remodeling becomes maladaptive and the previously beneficial
compensatory mechanisms become detrimental. Further preload Altered Excitation‐Contraction Coupling
expansion no longer enhances ventricular performance on the flat Calcium is a critical ion in regulating myocardial contrac-
portion of the Frank–Starling curve and instead congestion
develops. Increases in afterload depress ventricular performance tion and relaxation. Alterations in calcium conductance
even further down the Frank–Starling curves. across the sarcolemma, release from the sarcoplasmic