Page 35 - Heart Transplant Protocol
P. 35
Heart Function Service: Heart Transplant Protocols
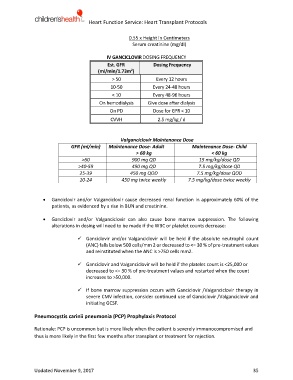
0.55 x Height in Centimeters
Serum creatinine (mg/dl)
IV GANCICLOVIR DOSING FREQUENCY
Est. GFR Dosing Frequency
2
(ml/min/1.73m )
> 50 Every 12 hours
10-50 Every 24-48 hours
< 10 Every 48-96 hours
On hemodialysis Give dose after dialysis
On PD Dose for GFR < 10
CVVH 2.5 mg/kg / d
Valganciclovir Maintenance Dose
GFR (ml/min) Maintenance Dose- Adult Maintenance Dose- Child
> 60 kg < 60 kg
>60 900 mg QD 15 mg/kg/dose QD
>40-59 450 mg QD 7.5 mg/kg/dose QD
25-39 450 mg QOD 7.5 mg/kg/dose QOD
10-24 450 mg twice weekly 7.5 mg/kg/dose twice weekly
Ganciclovir and/or Valganciclovir cause decreased renal function in approximately 60% of the
patients, as evidenced by a rise in BUN and creatinine.
Ganciclovir and/or Valganciclovir can also cause bone marrow suppression. The following
alterations in dosing will need to be made if the WBC or platelet counts decrease:
Ganciclovir and/or Valganciclovir will be held if the absolute neutrophil count
(ANC) falls below 500 cells/mm 2 or decreased to <= 30 % of pre-treatment values
and reinstituted when the ANC is >750 cells mm2.
Ganciclovir and Valganciclovir will be held if the platelet count is <25,000 or
decreased to <= 30 % of pre-treatment values and restarted when the count
increases to >50,000.
If bone marrow suppression occurs with Ganciclovir /Valganciclovir therapy in
severe CMV infection, consider continued use of Ganciclovir /Valganciclovir and
initiating GCSF.
Pneumocystis carinii pneumonia (PCP) Prophylaxis Protocol
Rationale: PCP is uncommon but is more likely when the patient is severely immunocompromised and
thus is more likely in the first few months after transplant or treatment for rejection.
Updated November 9, 2017 35