Page 100 - Chemistry
P. 100
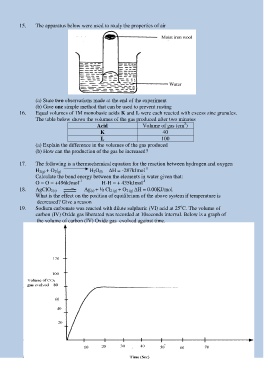
15. The apparatus below were used to study the properties of air
(a) State two observations made at the end of the experiment
(b) Give one simple method that can be used to prevent rusting
16. Equal volumes of 1M monobasic acids K and L were each reacted with excess zinc granules.
The table below shows the volumes of the gas produced after two minutes
3
Acid Volume of gas (cm )
K 40
L 100
(a) Explain the difference in the volumes of the gas produced
(b) How can the production of the gas be increased?
17. The following is a thermochemical equation for the reaction between hydrogen and oxygen
-1
H 2(g) + O 2( g) H 2O (l) H = -287kJmol
Calculate the bond energy between the elements in water given that:
-1
-1
O = O = +496kJmol H-H = + 435kJmol
18. AgClO 2(s) Ag (s) + ½ Cl 2 (g) + O 2 (g) H = 0.00KJ/mol
What is the effect on the position of equilibrium of the above system if temperature is
decreased? Give a reason
o
19. Sodium carbonate was reacted with dilute sulphuric (VI) acid at 25 C. The volume of
carbon (IV) Oxide gas liberated was recorded at 10seconds interval. Below is a graph of
the volume of carbon (IV) Oxide gas evolved against time.
www.kcse-online.info 99