Page 19 - GP Fall 2025
P. 19
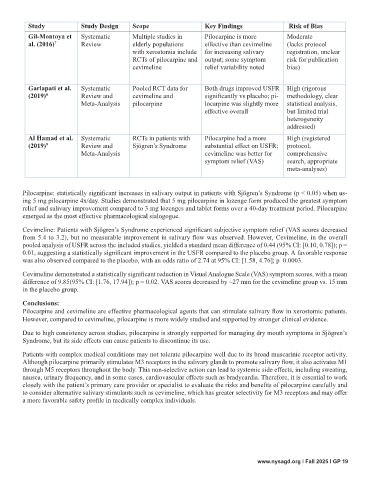
Study Study Design Scope Key Findings Risk of Bias
Gil-Montoya et Systematic Multiple studies in Pilocarpine is more Moderate
al. (2016) 7 Review elderly populations effective than cevimeline (lacks protocol
with xerostomia include for increasing salivary registration, unclear
RCTs of pilocarpine and output; some symptom risk for publication
cevimeline relief variability noted bias)
Garlapati et al. Systematic Pooled RCT data for Both drugs improved USFR High (rigorous
(2019) 8 Review and cevimeline and significantly vs placebo; pi- methodology, clear
Meta-Analysis pilocarpine locarpine was slightly more statistical analysis,
effective overall but limited trial
heterogeneity
addressed)
Al Hamad et al. Systematic RCTs in patients with Pilocarpine had a more High (registered
(2019) 9 Review and Sjögren’s Syndrome substantial effect on USFR; protocol,
Meta-Analysis cevimeline was better for comprehensive
symptom relief (VAS) search, appropriate
meta-analyses)
Pilocarpine: statistically significant increases in salivary output in patients with Sjögren’s Syndrome (p < 0.05) when us-
ing 5 mg pilocarpine 4x/day. Studies demonstrated that 5 mg pilocarpine in lozenge form produced the greatest symptom
relief and salivary improvement compared to 3 mg lozenges and tablet forms over a 40-day treatment period. Pilocarpine
emerged as the most effective pharmacological sialogogue.
Cevimeline: Patients with Sjögren’s Syndrome experienced significant subjective symptom relief (VAS scores decreased
from 5.4 to 3.2), but no measurable improvement in salivary flow was observed. However, Cevimeline, in the overall
pooled analysis of USFR across the included studies, yielded a standard mean difference of 0.44 (95% CI: [0.10, 0.78]); p =
0.01, suggesting a statistically significant improvement in the USFR compared to the placebo group. A favorable response
was also observed compared to the placebo, with an odds ratio of 2.74 at 95% CI: [1.58, 4.76]; p 0.0003.
Cevimeline demonstrated a statistically significant reduction in Visual Analogue Scale (VAS) symptom scores, with a mean
difference of 9.85(95% CI: [1.76, 17.94]); p = 0.02. VAS scores decreased by ~27 mm for the cevimeline group vs. 15 mm
in the placebo group.
Conclusions:
Pilocarpine and cevimeline are effective pharmacological agents that can stimulate salivary flow in xerostomic patients.
However, compared to cevimeline, pilocarpine is more widely studied and supported by stronger clinical evidence.
Due to high consistency across studies, pilocarpine is strongly supported for managing dry mouth symptoms in Sjögren’s
Syndrome, but its side effects can cause patients to discontinue its use.
Patients with complex medical conditions may not tolerate pilocarpine well due to its broad muscarinic receptor activity.
Although pilocarpine primarily stimulates M3 receptors in the salivary glands to promote salivary flow, it also activates M1
through M5 receptors throughout the body. This non-selective action can lead to systemic side effects, including sweating,
nausea, urinary frequency, and in some cases, cardiovascular effects such as bradycardia. Therefore, it is essential to work
closely with the patient’s primary care provider or specialist to evaluate the risks and benefits of pilocarpine carefully and
to consider alternative salivary stimulants such as cevimeline, which has greater selectivity for M3 receptors and may offer
a more favorable safety profile in medically complex individuals.
www.nysagd.org l Fall 2025 l GP 19