Page 119 - Microsoft Word - LessonPlan-Overview.doc
P. 119
all matter is made up of atoms, but Although atoms are mostly empty
all matter is made up of some kind space, at the outer edge of the
of particle. ‘empty space’ lies an electron
cloud. If an atom were blown up to
Most of the ordinary matter you
be the size of the earth, the
and I have experience with is made
nucleus would be the size of a
up of quarks and electrons, but
basketball at the Earth’s core with
most of the matter in the universe
the electron cloud on the outer
is actually dark matter (and we’re
surface. Electrons have very low
still working on defining what
mass and carry a negative charge.
exactly dark matter is).
Most atoms carry enough protons
This chemistry unit deals with to cancel out the negative charge
regular, ordinary matter – the kind of the electrons, giving the atom a
that tables, penguins, trees, and
net charge of zero (which is great,
books are made of... which is
otherwise it would be like a very
atoms. bad static-cling day… all day, every
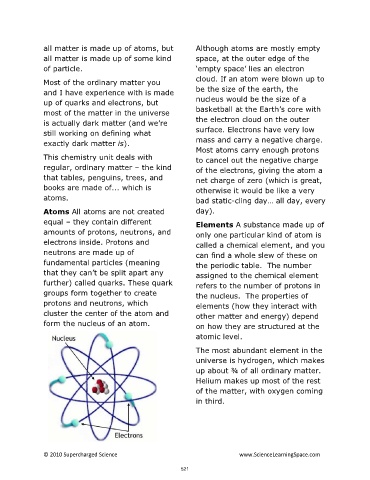
Atoms All atoms are not created day).
equal – they contain different
Elements A substance made up of
amounts of protons, neutrons, and
only one particular kind of atom is
electrons inside. Protons and
called a chemical element, and you
neutrons are made up of can find a whole slew of these on
fundamental particles (meaning the periodic table. The number
that they can’t be split apart any
assigned to the chemical element
further) called quarks. These quark
refers to the number of protons in
groups form together to create the nucleus. The properties of
protons and neutrons, which
elements (how they interact with
cluster the center of the atom and
other matter and energy) depend
form the nucleus of an atom.
on how they are structured at the
atomic level.
The most abundant element in the
universe is hydrogen, which makes
up about ¾ of all ordinary matter.
Helium makes up most of the rest
of the matter, with oxygen coming
in third.
© 2010 Supercharged Science www.ScienceLearningSpace.com
521