Page 501 - The ROV Manual - A User Guide for Remotely Operated Vehicles 2nd edition
P. 501
496 CHAPTER 18 Ancillary Sensors
It is often said that electricity is lazy, that is, it will take the path of least resistance, making the local electrochemical cell reactive to the area of highest potential/gradient. It is possible to change the local gradient by electrically attaching a more reactive metal to a less reactive metal, thus draw- ing the electrical reaction to the area of highest potential/gradient. This is known as “sacrificial anode” cathodic protection (SACP).
Sacrificial anode cathodic protection
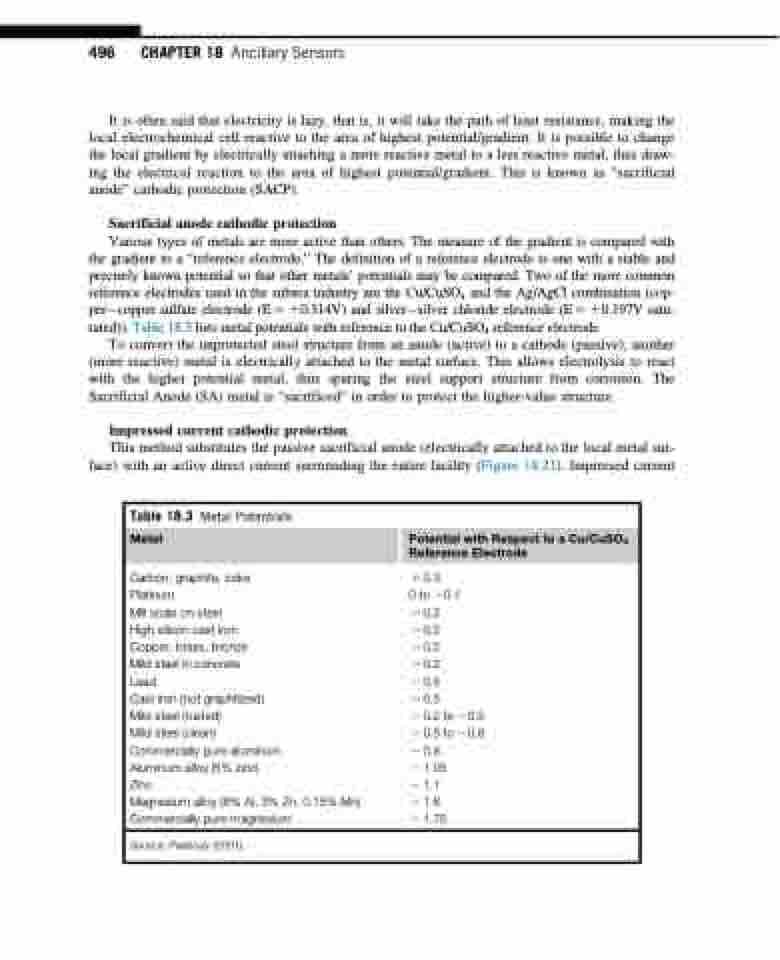
Various types of metals are more active than others. The measure of the gradient is compared with the gradient to a “reference electrode.” The definition of a reference electrode is one with a stable and precisely known potential so that other metals’ potentials may be compared. Two of the more common reference electrodes used in the subsea industry are the Cu/CuSO4 and the Ag/AgCl combination (cop- percopper sulfate electrode (E 5 10.314V) and silversilver chloride electrode (E 5 10.197V satu- rated)). Table 18.3 lists metal potentials with reference to the Cu/CuSO4 reference electrode.
To convert the unprotected steel structure from an anode (active) to a cathode (passive), another (more reactive) metal is electrically attached to the metal surface. This allows electrolysis to react with the higher potential metal, thus sparing the steel support structure from corrosion. The Sacrificial Anode (SA) metal is “sacrificed” in order to protect the higher-value structure.
Impressed current cathodic protection
This method substitutes the passive sacrificial anode (electrically attached to the local metal sur- face) with an active direct current surrounding the entire facility (Figure 18.21). Impressed current
Table 18.3 Metal Potentials
Metal Potential with Respect to a Cu/CuSO4 Reference Electrode
Carbon, graphite, coke Platinum
Mill scale on steel
High silicon cast iron Copper, brass, bronze Mild steel in concrete Lead
Cast iron (not graphitized) Mild steel (rusted)
Mild steel (clean) Commercially pure aluminum Aluminum alloy (5% zinc) Zinc
Magnesium alloy (6% Al, 3% Zn, 0.15% Mn) Commercially pure magnesium
1 0.3
0 to 20.1
2 0.2
2 0.2
2 0.2
2 0.2
2 0.5
2 0.5
2 0.2 to 20.5 2 0.5 to 20.8 2 0.8
2 1.05 2 1.1 2 1.6 2 1.75
Source: Peabody (2001).