Page 347 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 347
CHAPTER 18 The Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds 333
O HO
COOH
COOH
HO
HO
OH OH
Alprostadil Prostaglandin F 2`
(prostaglandin E ) (PGF 2` )
1
OH
COOH
O
COOCH 3
HO
CH 3
H C OH
3
OH Carboprost tromethamine
HO
(prostaglandin F 2` analog)
Misoprostol
(prostaglandin E analog)
1
COOH
O O
COOH
HO HO
OH OH
Dinoprostone Epoprostenol
(prostaglandin E , PGE ) (prostacyclin, PGI )
2
2
2
OH COOH
H
OH Iloprost
CH 3
H
–
OCH CO Na +
2
2
Treprostinil sodium OH OH
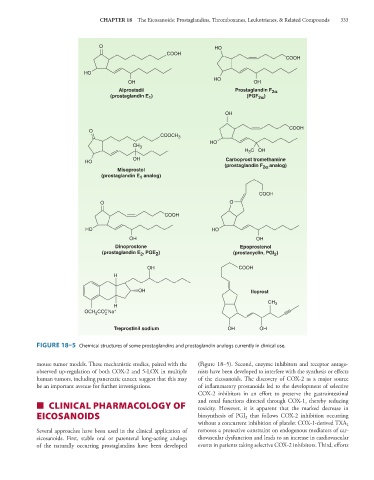
FIGURE 18–5 Chemical structures of some prostaglandins and prostaglandin analogs currently in clinical use.
mouse tumor models. These mechanistic studies, paired with the (Figure 18–5). Second, enzyme inhibitors and receptor antago-
observed up-regulation of both COX-2 and 5-LOX in multiple nists have been developed to interfere with the synthesis or effects
human tumors, including pancreatic cancer, suggest that this may of the eicosanoids. The discovery of COX-2 as a major source
be an important avenue for further investigations. of inflammatory prostanoids led to the development of selective
COX-2 inhibitors in an effort to preserve the gastrointestinal
■ CLINICAL PHARMACOLOGY OF and renal functions directed through COX-1, thereby reducing
toxicity. However, it is apparent that the marked decrease in
EICOSANOIDS biosynthesis of PGI that follows COX-2 inhibition occurring
2
without a concurrent inhibition of platelet COX-1-derived TXA
2
Several approaches have been used in the clinical application of removes a protective constraint on endogenous mediators of car-
eicosanoids. First, stable oral or parenteral long-acting analogs diovascular dysfunction and leads to an increase in cardiovascular
of the naturally occurring prostaglandins have been developed events in patients taking selective COX-2 inhibitors. Third, efforts