Page 397 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 397
CHAPTER 22 Sedative-Hypnotic Drugs 383
O
HN
1
1 6 R : R : CH 2 CH 3 R : CH 2 CH CH 2 R : CH 2 CH 3
1
1
1
O 2 5
3 4 R : R 2 : CH CH CH CH R 2 : CH CH CH CH :
HN 2 2 2 3 2 2 3 R 2
CH 3 CH 3
O
Barbiturate nucleus Pentobarbital Secobarbital Phenobarbital
C H O C H O
2 5
3 7
O HO
H N C O CH 2 C CH 2 O C NH 2 CH CCl 3
2
HN HO
O CH 3
Glutethimide Meprobamate Chloral hydrate
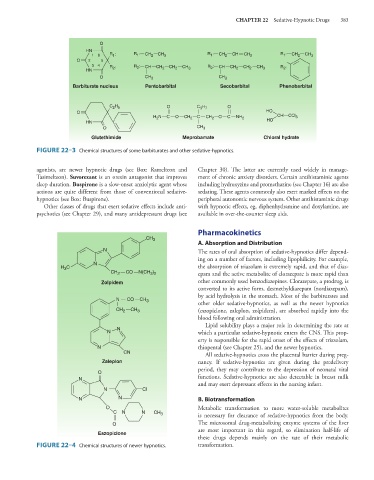
FIGURE 22–3 Chemical structures of some barbiturates and other sedative-hypnotics.
agonists, are newer hypnotic drugs (see Box: Ramelteon and Chapter 30). The latter are currently used widely in manage-
Tasimelteon). Suvorexant is an orexin antagonist that improves ment of chronic anxiety disorders. Certain antihistaminic agents
sleep duration. Buspirone is a slow-onset anxiolytic agent whose including hydroxyzine and promethazine (see Chapter 16) are also
actions are quite different from those of conventional sedative- sedating. These agents commonly also exert marked effects on the
hypnotics (see Box: Buspirone). peripheral autonomic nervous system. Other antihistaminic drugs
Other classes of drugs that exert sedative effects include anti- with hypnotic effects, eg, diphenhydramine and doxylamine, are
psychotics (see Chapter 29), and many antidepressant drugs (see available in over-the-counter sleep aids.
Pharmacokinetics
CH 3
A. Absorption and Distribution
N The rates of oral absorption of sedative-hypnotics differ depend-
ing on a number of factors, including lipophilicity. For example,
N
H C the absorption of triazolam is extremely rapid, and that of diaz-
3
CH 2 CO N(CH ) epam and the active metabolite of clorazepate is more rapid than
3 2
Zolpidem other commonly used benzodiazepines. Clorazepate, a prodrug, is
converted to its active form, desmethyldiazepam (nordiazepam),
by acid hydrolysis in the stomach. Most of the barbiturates and
N CO CH 3 other older sedative-hypnotics, as well as the newer hypnotics
CH 2 CH 3 (eszopiclone, zaleplon, zolpidem), are absorbed rapidly into the
blood following oral administration.
Lipid solubility plays a major role in determining the rate at
N
N which a particular sedative-hypnotic enters the CNS. This prop-
erty is responsible for the rapid onset of the effects of triazolam,
N thiopental (see Chapter 25), and the newer hypnotics.
CN All sedative-hypnotics cross the placental barrier during preg-
Zaleplon nancy. If sedative-hypnotics are given during the predelivery
period, they may contribute to the depression of neonatal vital
O
N functions. Sedative-hypnotics are also detectable in breast milk
and may exert depressant effects in the nursing infant.
N Cl
N N B. Biotransformation
O Metabolic transformation to more water-soluble metabolites
C N N CH 3 is necessary for clearance of sedative-hypnotics from the body.
O The microsomal drug-metabolizing enzyme systems of the liver
are most important in this regard, so elimination half-life of
Eszopiclone
these drugs depends mainly on the rate of their metabolic
FIGURE 22–4 Chemical structures of newer hypnotics. transformation.