Page 399 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 399
CHAPTER 22 Sedative-Hypnotic Drugs 385
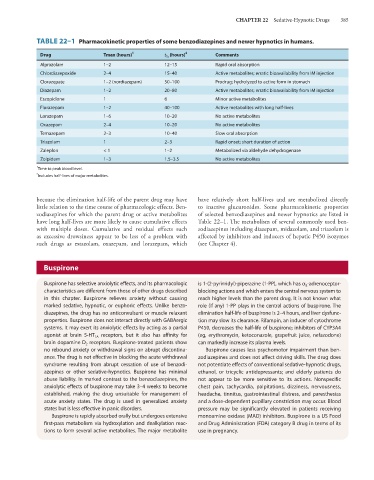
TABLE 22–1 Pharmacokinetic properties of some benzodiazepines and newer hypnotics in humans.
Drug Tmax (hours) 1 t ½ (hours) 2 Comments
Alprazolam 1–2 12–15 Rapid oral absorption
Chlordiazepoxide 2–4 15–40 Active metabolites; erratic bioavailability from IM injection
Clorazepate 1–2 (nordiazepam) 50–100 Prodrug; hydrolyzed to active form in stomach
Diazepam 1–2 20–80 Active metabolites; erratic bioavailability from IM injection
Eszopiclone 1 6 Minor active metabolites
Flurazepam 1–2 40–100 Active metabolites with long half-lives
Lorazepam 1–6 10–20 No active metabolites
Oxazepam 2–4 10–20 No active metabolites
Temazepam 2–3 10–40 Slow oral absorption
Triazolam 1 2–3 Rapid onset; short duration of action
Zaleplon < 1 1–2 Metabolized via aldehyde dehydrogenase
Zolpidem 1–3 1.5–3.5 No active metabolites
1 Time to peak blood level.
2
Includes half-lives of major metabolites.
because the elimination half-life of the parent drug may have have relatively short half-lives and are metabolized directly
little relation to the time course of pharmacologic effects. Ben- to inactive glucuronides. Some pharmacokinetic properties
zodiazepines for which the parent drug or active metabolites of selected benzodiazepines and newer hypnotics are listed in
have long half-lives are more likely to cause cumulative effects Table 22–1. The metabolism of several commonly used ben-
with multiple doses. Cumulative and residual effects such zodiazepines including diazepam, midazolam, and triazolam is
as excessive drowsiness appear to be less of a problem with affected by inhibitors and inducers of hepatic P450 isozymes
such drugs as estazolam, oxazepam, and lorazepam, which (see Chapter 4).
Buspirone
Buspirone has selective anxiolytic effects, and its pharmacologic is 1-(2-pyrimidyl)-piperazine (1-PP), which has α 2 -adrenoceptor-
characteristics are different from those of other drugs described blocking actions and which enters the central nervous system to
in this chapter. Buspirone relieves anxiety without causing reach higher levels than the parent drug. It is not known what
marked sedative, hypnotic, or euphoric effects. Unlike benzo- role (if any) 1-PP plays in the central actions of buspirone. The
diazepines, the drug has no anticonvulsant or muscle relaxant elimination half-life of buspirone is 2–4 hours, and liver dysfunc-
properties. Buspirone does not interact directly with GABAergic tion may slow its clearance. Rifampin, an inducer of cytochrome
systems. It may exert its anxiolytic effects by acting as a partial P450, decreases the half-life of buspirone; inhibitors of CYP3A4
agonist at brain 5-HT 1A receptors, but it also has affinity for (eg, erythromycin, ketoconazole, grapefruit juice, nefazodone)
brain dopamine D 2 receptors. Buspirone-treated patients show can markedly increase its plasma levels.
no rebound anxiety or withdrawal signs on abrupt discontinu- Buspirone causes less psychomotor impairment than ben-
ance. The drug is not effective in blocking the acute withdrawal zodiazepines and does not affect driving skills. The drug does
syndrome resulting from abrupt cessation of use of benzodi- not potentiate effects of conventional sedative-hypnotic drugs,
azepines or other sedative-hypnotics. Buspirone has minimal ethanol, or tricyclic antidepressants; and elderly patients do
abuse liability. In marked contrast to the benzodiazepines, the not appear to be more sensitive to its actions. Nonspecific
anxiolytic effects of buspirone may take 3–4 weeks to become chest pain, tachycardia, palpitations, dizziness, nervousness,
established, making the drug unsuitable for management of headache, tinnitus, gastrointestinal distress, and paresthesias
acute anxiety states. The drug is used in generalized anxiety and a dose-dependent pupillary constriction may occur. Blood
states but is less effective in panic disorders. pressure may be significantly elevated in patients receiving
Buspirone is rapidly absorbed orally but undergoes extensive monoamine oxidase (MAO) inhibitors. Buspirone is a US Food
first-pass metabolism via hydroxylation and dealkylation reac- and Drug Administration (FDA) category B drug in terms of its
tions to form several active metabolites. The major metabolite use in pregnancy.