Page 459 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 459
CHAPTER 25 General Anesthetics 445
blood concentration initially is very low, and equilibrium with the B. Elimination
alveolar space is achieved slowly. Recovery from inhalation anesthesia follows some of the same
During maintenance of anesthesia with inhaled anesthetics, principles in reverse that are important during induction. The
the drug continues to be transferred between various tissues at time to recovery from inhalation anesthesia depends on the rate
rates dependent on the solubility of the agent, the concentration of elimination of the anesthetic from the brain. One of the most
gradient between the blood and the respective tissue, and the important factors governing rate of recovery is the blood:gas parti-
tissue blood flow. Although muscle and skin constitute 50% of tion coefficient of the anesthetic agent. When the anesthesiologist
the total body mass, anesthetics accumulate more slowly in these discontinues the administration of the anesthetic agent to the
tissues than in highly perfused tissues (eg, brain) because they lung, the alveolar concentration falls rapidly. Insoluble anesthetics
receive only one fifth of the resting cardiac output. Although most that prefer the gas phase over blood will then rapidly diffuse into
anesthetic agents are highly soluble in adipose (fatty) tissues, the the alveolus and be removed from the body by the process of lung
relatively low blood perfusion to these tissues delays accumula- ventilation. Other factors controlling rate of recovery include pul-
tion, and equilibrium is unlikely to occur with most anesthetics monary blood flow and tissue solubility of the anesthetic.
during a typical 1- to 3-hour operation. Two features differentiate the recovery phase from the induc-
The combined effect of ventilation, solubility in the dif- tion phase. First, transfer of an anesthetic from the lungs to blood
ferent tissues, cardiac output, and blood flow distribution during induction can be enhanced by increasing its concentration
determines the rate of rise of F /F characteristic of each drug. in inspired air, but the reverse transfer process cannot be enhanced
I
A
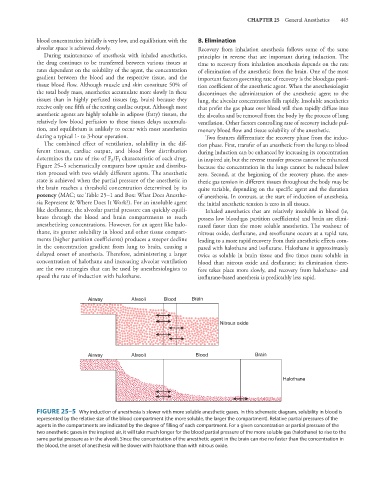
Figure 25–5 schematically compares how uptake and distribu- because the concentration in the lungs cannot be reduced below
tion proceed with two widely different agents. The anesthetic zero. Second, at the beginning of the recovery phase, the anes-
state is achieved when the partial pressure of the anesthetic in thetic gas tension in different tissues throughout the body may be
the brain reaches a threshold concentration determined by its quite variable, depending on the specific agent and the duration
potency (MAC; see Table 25–1 and Box: What Does Anesthe- of anesthesia. In contrast, at the start of induction of anesthesia,
sia Represent & Where Does It Work?). For an insoluble agent the initial anesthetic tension is zero in all tissues.
like desflurane, the alveolar partial pressure can quickly equili- Inhaled anesthetics that are relatively insoluble in blood (ie,
brate through the blood and brain compartments to reach possess low blood:gas partition coefficients) and brain are elimi-
anesthetizing concentrations. However, for an agent like halo- nated faster than the more soluble anesthetics. The washout of
thane, its greater solubility in blood and other tissue compart- nitrous oxide, desflurane, and sevoflurane occurs at a rapid rate,
ments (higher partition coefficients) produces a steeper decline leading to a more rapid recovery from their anesthetic effects com-
in the concentration gradient from lung to brain, causing a pared with halothane and isoflurane. Halothane is approximately
delayed onset of anesthesia. Therefore, administering a larger twice as soluble in brain tissue and five times more soluble in
concentration of halothane and increasing alveolar ventilation blood than nitrous oxide and desflurane; its elimination there-
are the two strategies that can be used by anesthesiologists to fore takes place more slowly, and recovery from halothane- and
speed the rate of induction with halothane. isoflurane-based anesthesia is predictably less rapid.
Airway Alveoli Blood Brain
Nitrous oxide
Airway Alveoli Blood Brain
Halothane
FIGURE 25–5 Why induction of anesthesia is slower with more soluble anesthetic gases. In this schematic diagram, solubility in blood is
represented by the relative size of the blood compartment (the more soluble, the larger the compartment). Relative partial pressures of the
agents in the compartments are indicated by the degree of filling of each compartment. For a given concentration or partial pressure of the
two anesthetic gases in the inspired air, it will take much longer for the blood partial pressure of the more soluble gas (halothane) to rise to the
same partial pressure as in the alveoli. Since the concentration of the anesthetic agent in the brain can rise no faster than the concentration in
the blood, the onset of anesthesia will be slower with halothane than with nitrous oxide.