Page 607 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 607
CHAPTER 33 Agents Used in Cytopenias; Hematopoietic Growth Factors 593
4
Spleen, other tissues
Blood
macrophage
Senescent
RBC
1
Gut Intestinal epithelial cells
lumen Hgb
Hgb Hgb F
HCP1 Tf
FP
DB F FP –
Fe 3+
Hepcidin
FP
Fe 2+ DMT1
– –
Hepcidin
– F
TfR
Erythroferrone
Heme
RBC
production
Fe
2 TfR
Bone marrow 3
erythrocyte precursor Hepatocyte
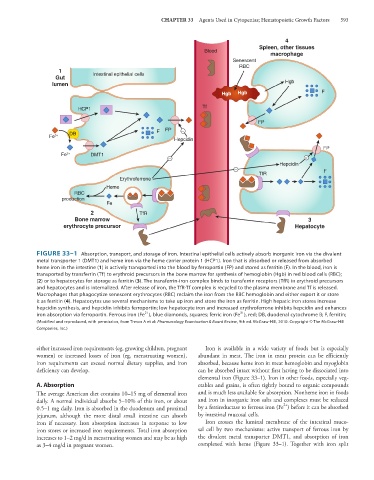
FIGURE 33–1 Absorption, transport, and storage of iron. Intestinal epithelial cells actively absorb inorganic iron via the divalent
metal transporter 1 (DMT1) and heme iron via the heme carrier protein 1 (HCP1). Iron that is absorbed or released from absorbed
heme iron in the intestine (1) is actively transported into the blood by ferroportin (FP) and stored as ferritin (F). In the blood, iron is
transported by transferrin (Tf) to erythroid precursors in the bone marrow for synthesis of hemoglobin (Hgb) in red blood cells (RBC);
(2) or to hepatocytes for storage as ferritin (3). The transferrin-iron complex binds to transferrin receptors (TfR) in erythroid precursors
and hepatocytes and is internalized. After release of iron, the TfR-Tf complex is recycled to the plasma membrane and Tf is released.
Macrophages that phagocytize senescent erythrocytes (RBC) reclaim the iron from the RBC hemoglobin and either export it or store
it as ferritin (4). Hepatocytes use several mechanisms to take up iron and store the iron as ferritin. High hepatic iron stores increase
hepcidin synthesis, and hepcidin inhibits ferroportin; low hepatocyte iron and increased erythroferrone inhibits hepcidin and enhances
3+
2+
iron absorption via ferroportin. Ferrous iron (Fe ), blue diamonds, squares; ferric iron (Fe ), red; DB, duodenal cytochrome B; F, ferritin;
(Modified and reproduced, with permission, from Trevor A et al: Pharmacology Examination & Board Review, 9th ed. McGraw-Hill, 2010. Copyright © The McGraw-Hill
Companies, Inc.)
either increased iron requirements (eg, growing children, pregnant Iron is available in a wide variety of foods but is especially
women) or increased losses of iron (eg, menstruating women), abundant in meat. The iron in meat protein can be efficiently
iron requirements can exceed normal dietary supplies, and iron absorbed, because heme iron in meat hemoglobin and myoglobin
deficiency can develop. can be absorbed intact without first having to be dissociated into
elemental iron (Figure 33–1). Iron in other foods, especially veg-
A. Absorption etables and grains, is often tightly bound to organic compounds
The average American diet contains 10–15 mg of elemental iron and is much less available for absorption. Nonheme iron in foods
daily. A normal individual absorbs 5–10% of this iron, or about and iron in inorganic iron salts and complexes must be reduced
2+
0.5–1 mg daily. Iron is absorbed in the duodenum and proximal by a ferrireductase to ferrous iron (Fe ) before it can be absorbed
jejunum, although the more distal small intestine can absorb by intestinal mucosal cells.
iron if necessary. Iron absorption increases in response to low Iron crosses the luminal membrane of the intestinal muco-
iron stores or increased iron requirements. Total iron absorption sal cell by two mechanisms: active transport of ferrous iron by
increases to 1–2 mg/d in menstruating women and may be as high the divalent metal transporter DMT1, and absorption of iron
as 3–4 mg/d in pregnant women. complexed with heme (Figure 33–1). Together with iron split