Page 608 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 608
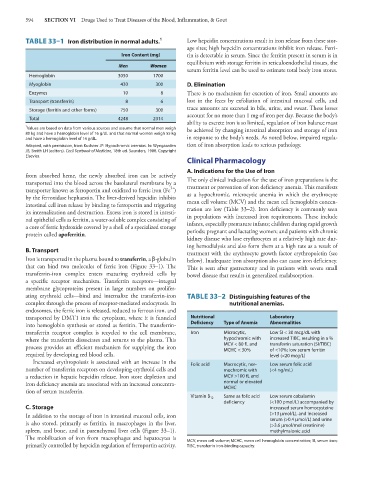
594 SECTION VI Drugs Used to Treat Diseases of the Blood, Inflammation, & Gout
TABLE 33–1 Iron distribution in normal adults. 1 Low hepcidin concentrations result in iron release from these stor-
age sites; high hepcidin concentrations inhibit iron release. Ferri-
Iron Content (mg) tin is detectable in serum. Since the ferritin present in serum is in
equilibrium with storage ferritin in reticuloendothelial tissues, the
Men Women
serum ferritin level can be used to estimate total body iron stores.
Hemoglobin 3050 1700
Myoglobin 430 300 D. Elimination
Enzymes 10 8 There is no mechanism for excretion of iron. Small amounts are
Transport (transferrin) 8 6 lost in the feces by exfoliation of intestinal mucosal cells, and
Storage (ferritin and other forms) 750 300 trace amounts are excreted in bile, urine, and sweat. These losses
account for no more than 1 mg of iron per day. Because the body’s
Total 4248 2314
ability to excrete iron is so limited, regulation of iron balance must
1 Values are based on data from various sources and assume that normal men weigh be achieved by changing intestinal absorption and storage of iron
80 kg and have a hemoglobin level of 16 g/dL and that normal women weigh 55 kg
and have a hemoglobin level of 14 g/dL. in response to the body’s needs. As noted below, impaired regula-
Adapted, with permission, from Kushner JP: Hypochromic anemias. In: Wyngaarden tion of iron absorption leads to serious pathology.
JB, Smith LH (editors). Cecil Textbook of Medicine, 18th ed. Saunders, 1988. Copyright
Elsevier.
Clinical Pharmacology
A. Indications for the Use of Iron
from absorbed heme, the newly absorbed iron can be actively
transported into the blood across the basolateral membrane by a The only clinical indication for the use of iron preparations is the
3+
transporter known as ferroportin and oxidized to ferric iron (Fe ) treatment or prevention of iron deficiency anemia. This manifests
by the ferroxidase hephaestin. The liver-derived hepcidin inhibits as a hypochromic, microcytic anemia in which the erythrocyte
intestinal cell iron release by binding to ferroportin and triggering mean cell volume (MCV) and the mean cell hemoglobin concen-
its internalization and destruction. Excess iron is stored in intesti- tration are low (Table 33–2). Iron deficiency is commonly seen
nal epithelial cells as ferritin, a water-soluble complex consisting of in populations with increased iron requirements. These include
a core of ferric hydroxide covered by a shell of a specialized storage infants, especially premature infants; children during rapid growth
protein called apoferritin. periods; pregnant and lactating women; and patients with chronic
kidney disease who lose erythrocytes at a relatively high rate dur-
ing hemodialysis and also form them at a high rate as a result of
B. Transport treatment with the erythrocyte growth factor erythropoietin (see
Iron is transported in the plasma bound to transferrin, a β-globulin below). Inadequate iron absorption also can cause iron deficiency.
that can bind two molecules of ferric iron (Figure 33–1). The This is seen after gastrectomy and in patients with severe small
transferrin-iron complex enters maturing erythroid cells by bowel disease that results in generalized malabsorption.
a specific receptor mechanism. Transferrin receptors—integral
membrane glycoproteins present in large numbers on prolifer-
ating erythroid cells—bind and internalize the transferrin-iron TABLE 33–2 Distinguishing features of the
complex through the process of receptor-mediated endocytosis. In nutritional anemias.
endosomes, the ferric iron is released, reduced to ferrous iron, and
transported by DMT1 into the cytoplasm, where it is funneled Nutritional Laboratory
into hemoglobin synthesis or stored as ferritin. The transferrin- Deficiency Type of Anemia Abnormalities
transferrin receptor complex is recycled to the cell membrane, Iron Microcytic, Low SI < 30 mcg/dL with
where the transferrin dissociates and returns to the plasma. This hypochromic with increased TIBC, resulting in a %
process provides an efficient mechanism for supplying the iron MCV < 80 fL and transferrin saturation (SI/TIBC)
of <10%; low serum ferritin
MCHC < 30%
required by developing red blood cells. level (<20 mcg/L)
Increased erythropoiesis is associated with an increase in the Folic acid Macrocytic, nor- Low serum folic acid
number of transferrin receptors on developing erythroid cells and mochromic with (<4 ng/mL)
a reduction in hepatic hepcidin release. Iron store depletion and MCV >100 fL and
iron deficiency anemia are associated with an increased concentra- normal or elevated
tion of serum transferrin. MCHC
Vitamin B 12 Same as folic acid Low serum cobalamin
deficiency (<100 pmol/L) accompanied by
C. Storage increased serum homocysteine
In addition to the storage of iron in intestinal mucosal cells, iron (>13 μmol/L), and increased
is also stored, primarily as ferritin, in macrophages in the liver, serum (>0.4 μmol/L) and urine
(>3.6 μmol/mol creatinine)
spleen, and bone, and in parenchymal liver cells (Figure 33–1). methylmalonic acid
The mobilization of iron from macrophages and hepatocytes is MCV, mean cell volume; MCHC, mean cell hemoglobin concentration; SI, serum iron;
primarily controlled by hepcidin regulation of ferroportin activity. TIBC, transferrin iron-binding capacity.