Page 617 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 617
CHAPTER 33 Agents Used in Cytopenias; Hematopoietic Growth Factors 603
neutrophils. GM-CSF acts together with interleukin-2 to stimu- neutropenia; patients with a prior episode of febrile neutropenia
late T-cell proliferation and appears to be a locally active factor after cytotoxic chemotherapy; patients at high risk for febrile
at the site of inflammation. GM-CSF mobilizes peripheral blood neutropenia; and patients who are unlikely to survive an episode
stem cells, but it is significantly less efficacious and more toxic of febrile neutropenia. Pegfilgrastim is an alternative to G-CSF
than G-CSF in this regard. for prevention of chemotherapy-induced febrile neutropenia.
Pegfilgrastim can be administered once per chemotherapy cycle,
Clinical Pharmacology and it may shorten the period of severe neutropenia slightly more
than G-CSF.
A. Cancer Chemotherapy-Induced Neutropenia Like G-CSF and pegfilgrastim, GM-CSF also reduces the
Neutropenia is a common adverse effect of the cytotoxic drugs duration of neutropenia after cytotoxic chemotherapy. It has been
used to treat cancer and increases the risk of serious infection in more difficult to show that GM-CSF reduces the incidence of
patients receiving chemotherapy. Unlike the treatment of anemia febrile neutropenia, probably because GM-CSF itself can induce
and thrombocytopenia, transfusion of neutropenic patients with fever. In the treatment of chemotherapy-induced neutropenia,
granulocytes collected from donors is performed rarely and with G-CSF 5 mcg/kg daily or GM-CSF 250 mcg/m daily is usually
2
limited success. The introduction of G-CSF in 1991 represented a started within 24–72 hours after completing chemotherapy and
milestone in the treatment of chemotherapy-induced neutropenia. is continued until the absolute neutrophil count is greater than
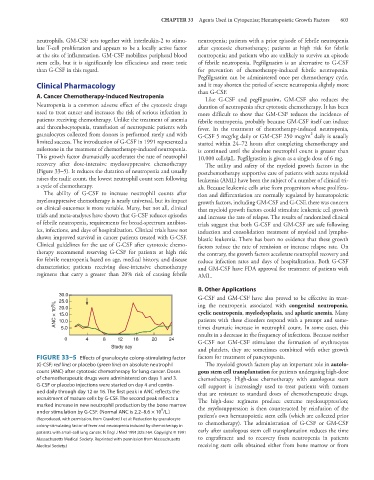
This growth factor dramatically accelerates the rate of neutrophil 10,000 cells/μL. Pegfilgrastim is given as a single dose of 6 mg.
recovery after dose-intensive myelosuppressive chemotherapy The utility and safety of the myeloid growth factors in the
(Figure 33–5). It reduces the duration of neutropenia and usually postchemotherapy supportive care of patients with acute myeloid
raises the nadir count, the lowest neutrophil count seen following leukemia (AML) have been the subject of a number of clinical tri-
a cycle of chemotherapy. als. Because leukemic cells arise from progenitors whose prolifera-
The ability of G-CSF to increase neutrophil counts after tion and differentiation are normally regulated by hematopoietic
myelosuppressive chemotherapy is nearly universal, but its impact growth factors, including GM-CSF and G-CSF, there was concern
on clinical outcomes is more variable. Many, but not all, clinical that myeloid growth factors could stimulate leukemic cell growth
trials and meta-analyses have shown that G-CSF reduces episodes and increase the rate of relapse. The results of randomized clinical
of febrile neutropenia, requirements for broad-spectrum antibiot- trials suggest that both G-CSF and GM-CSF are safe following
ics, infections, and days of hospitalization. Clinical trials have not induction and consolidation treatment of myeloid and lympho-
shown improved survival in cancer patients treated with G-CSF. blastic leukemia. There has been no evidence that these growth
Clinical guidelines for the use of G-CSF after cytotoxic chemo- factors reduce the rate of remission or increase relapse rate. On
therapy recommend reserving G-CSF for patients at high risk the contrary, the growth factors accelerate neutrophil recovery and
for febrile neutropenia based on age, medical history, and disease reduce infection rates and days of hospitalization. Both G-CSF
characteristics; patients receiving dose-intensive chemotherapy and GM-CSF have FDA approval for treatment of patients with
regimens that carry a greater than 20% risk of causing febrile AML.
B. Other Applications
30.0 G-CSF and GM-CSF have also proved to be effective in treat-
25.0 ing the neutropenia associated with congenital neutropenia,
ANC × 10 9 / L 10.0 cyclic neutropenia, myelodysplasia, and aplastic anemia. Many
20.0
15.0
patients with these disorders respond with a prompt and some-
times dramatic increase in neutrophil count. In some cases, this
5.0
results in a decrease in the frequency of infections. Because neither
0 4 8 12 16 20 24 G-CSF nor GM-CSF stimulates the formation of erythrocytes
Study day
and platelets, they are sometimes combined with other growth
FIGURE 33–5 Effects of granulocyte colony-stimulating factor factors for treatment of pancytopenia.
(G-CSF; red line) or placebo (green line) on absolute neutrophil The myeloid growth factors play an important role in autolo-
count (ANC) after cytotoxic chemotherapy for lung cancer. Doses gous stem cell transplantation for patients undergoing high-dose
of chemotherapeutic drugs were administered on days 1 and 3. chemotherapy. High-dose chemotherapy with autologous stem
G-CSF or placebo injections were started on day 4 and contin- cell support is increasingly used to treat patients with tumors
ued daily through day 12 or 16. The first peak in ANC reflects the that are resistant to standard doses of chemotherapeutic drugs.
recruitment of mature cells by G-CSF. The second peak reflects a The high-dose regimens produce extreme myelosuppression;
marked increase in new neutrophil production by the bone marrow the myelosuppression is then counteracted by reinfusion of the
9
under stimulation by G-CSF. (Normal ANC is 2.2–8.6 × 10 /L.) patient’s own hematopoietic stem cells (which are collected prior
(Reproduced, with permission, from Crawford J et al: Reduction by granulocyte
colony-stimulating factor of fever and neutropenia induced by chemotherapy in to chemotherapy). The administration of G-CSF or GM-CSF
patients with small-cell lung cancer. N Engl J Med 1991;325:164. Copyright © 1991 early after autologous stem cell transplantation reduces the time
Massachusetts Medical Society. Reprinted with permission from Massachusetts to engraftment and to recovery from neutropenia in patients
Medical Society.) receiving stem cells obtained either from bone marrow or from