Page 719 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 719
CHAPTER 39 Adrenocorticosteroids & Adrenocortical Antagonists 705
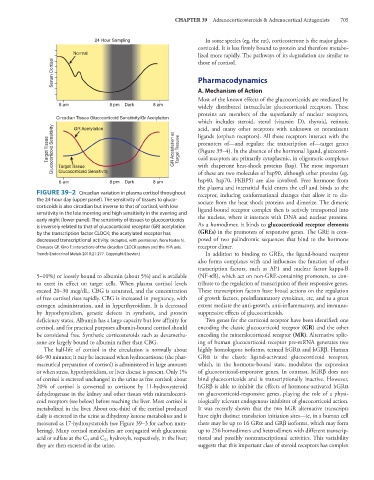
24 Hour Sampling In some species (eg, the rat), corticosterone is the major gluco-
corticoid. It is less firmly bound to protein and therefore metabo-
Normal lized more rapidly. The pathways of its degradation are similar to
Serum Cortisol Pharmacodynamics
those of cortisol.
A. Mechanism of Action
Most of the known effects of the glucocorticoids are mediated by
8 am 8 pm Dark 8 am widely distributed intracellular glucocorticoid receptors. These
proteins are members of the superfamily of nuclear receptors,
Circadian Tissue Glucocorticoid Sensitivity/Gr Acetylation
which includes steroid, sterol (vitamin D), thyroid, retinoic
acid, and many other receptors with unknown or nonexistent
GR Acetylation
Target Tissue Glucocorticoid Sensitivity GR Acetylation at Target Tissues ligands (orphan receptors). All these receptors interact with the
promoters of—and regulate the transcription of—target genes
(Figure 39–4). In the absence of the hormonal ligand, glucocorti-
coid receptors are primarily cytoplasmic, in oligomeric complexes
with chaperone heat-shock proteins (hsp). The most important
Target Tissue
Glucocorticoid Sensitivity
hsp40, hsp70, FKBP5) are also involved. Free hormone from
8 am 8 pm Dark 8 am of these are two molecules of hsp90, although other proteins (eg,
the plasma and interstitial fluid enters the cell and binds to the
FIGURE 39–2 Circadian variation in plasma cortisol throughout receptor, inducing conformational changes that allow it to dis-
the 24-hour day (upper panel). The sensitivity of tissues to gluco- sociate from the heat shock proteins and dimerize. The dimeric
corticoids is also circadian but inverse to that of cortisol, with low ligand-bound receptor complex then is actively transported into
sensitivity in the late morning and high sensitivity in the evening and
early night (lower panel). The sensitivity of tissues to glucocorticoids the nucleus, where it interacts with DNA and nuclear proteins.
is inversely related to that of glucocorticoid receptor (GR) acetylation As a homodimer, it binds to glucocorticoid receptor elements
by the transcription factor CLOCK; the acetylated receptor has (GREs) in the promoters of responsive genes. The GRE is com-
decreased transcriptional activity. (Adapted, with permission, from Nader N, posed of two palindromic sequences that bind to the hormone
Chrousos GP, Kino T: Interactions of the circadian CLOCK system and the HPA axis. receptor dimer.
Trends Endocrinol Metab 2010;21:277. Copyright Elsevier.) In addition to binding to GREs, the ligand-bound receptor
also forms complexes with and influences the function of other
transcription factors, such as AP1 and nuclear factor kappa-B
5–10%) or loosely bound to albumin (about 5%) and is available (NF-κB), which act on non-GRE-containing promoters, to con-
to exert its effect on target cells. When plasma cortisol levels tribute to the regulation of transcription of their responsive genes.
exceed 20–30 mcg/dL, CBG is saturated, and the concentration These transcription factors have broad actions on the regulation
of free cortisol rises rapidly. CBG is increased in pregnancy, with of growth factors, proinflammatory cytokines, etc, and to a great
estrogen administration, and in hyperthyroidism. It is decreased extent mediate the anti-growth, anti-inflammatory, and immuno-
by hypothyroidism, genetic defects in synthesis, and protein suppressive effects of glucocorticoids.
deficiency states. Albumin has a large capacity but low affinity for Two genes for the corticoid receptor have been identified: one
cortisol, and for practical purposes albumin-bound cortisol should encoding the classic glucocorticoid receptor (GR) and the other
be considered free. Synthetic corticosteroids such as dexametha- encoding the mineralocorticoid receptor (MR). Alternative splic-
sone are largely bound to albumin rather than CBG. ing of human glucocorticoid receptor pre-mRNA generates two
The half-life of cortisol in the circulation is normally about highly homologous isoforms, termed hGRα and hGRβ. Human
60–90 minutes; it may be increased when hydrocortisone (the phar- GRα is the classic ligand-activated glucocorticoid receptor,
maceutical preparation of cortisol) is administered in large amounts which, in the hormone-bound state, modulates the expression
or when stress, hypothyroidism, or liver disease is present. Only 1% of glucocorticoid-responsive genes. In contrast, hGRβ does not
of cortisol is excreted unchanged in the urine as free cortisol; about bind glucocorticoids and is transcriptionally inactive. However,
20% of cortisol is converted to cortisone by 11-hydroxysteroid hGRβ is able to inhibit the effects of hormone-activated hGRα
dehydrogenase in the kidney and other tissues with mineralocorti- on glucocorticoid-responsive genes, playing the role of a physi-
coid receptors (see below) before reaching the liver. Most cortisol is ologically relevant endogenous inhibitor of glucocorticoid action.
metabolized in the liver. About one-third of the cortisol produced It was recently shown that the two hGR alternative transcripts
daily is excreted in the urine as dihydroxy ketone metabolites and is have eight distinct translation initiation sites—ie, in a human cell
measured as 17-hydroxysteroids (see Figure 39–3 for carbon num- there may be up to 16 GRα and GRβ isoforms, which may form
bering). Many cortisol metabolites are conjugated with glucuronic up to 256 homodimers and heterodimers with different transcrip-
acid or sulfate at the C and C hydroxyls, respectively, in the liver; tional and possibly nontranscriptional activities. This variability
21
3
they are then excreted in the urine. suggests that this important class of steroid receptors has complex