Page 882 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 882
868 SECTION VIII Chemotherapeutic Drugs
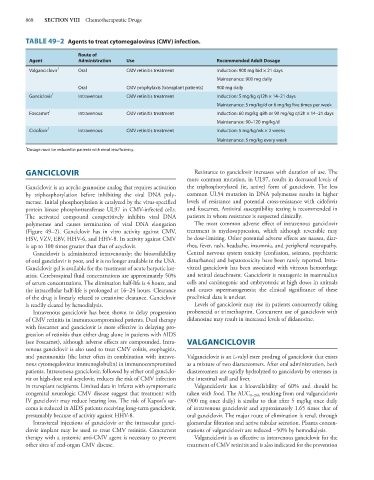
TABLE 49–2 Agents to treat cytomegalovirus (CMV) infection.
Route of
Agent Administration Use Recommended Adult Dosage
Valganciclovir 1 Oral CMV retinitis treatment Induction: 900 mg bid × 21 days
Maintenance: 900 mg daily
Oral CMV prophylaxis (transplant patients) 900 mg daily
Ganciclovir 1 Intravenous CMV retinitis treatment Induction: 5 mg/kg q12h × 14–21 days
Maintenance: 5 mg/kg/d or 6 mg/kg five times per week
Foscarnet 1 Intravenous CMV retinitis treatment Induction: 60 mg/kg q8h or 90 mg/kg q12h × 14–21 days
Maintenance: 90–120 mg/kg/d
Cidofovir 1 Intravenous CMV retinitis treatment Induction: 5 mg/kg/wk × 2 weeks
Maintenance: 5 mg/kg every week
1 Dosage must be reduced in patients with renal insufficiency.
GANCICLOVIR Resistance to ganciclovir increases with duration of use. The
more common mutation, in UL97, results in decreased levels of
Ganciclovir is an acyclic guanosine analog that requires activation the triphosphorylated (ie, active) form of ganciclovir. The less
by triphosphorylation before inhibiting the viral DNA poly- common UL54 mutation in DNA polymerase results in higher
merase. Initial phosphorylation is catalyzed by the virus-specified levels of resistance and potential cross-resistance with cidofovir
protein kinase phosphotransferase UL97 in CMV-infected cells. and foscarnet. Antiviral susceptibility testing is recommended in
The activated compound competitively inhibits viral DNA patients in whom resistance is suspected clinically.
polymerase and causes termination of viral DNA elongation The most common adverse effect of intravenous ganciclovir
(Figure 49–2). Ganciclovir has in vitro activity against CMV, treatment is myelosuppression, which although reversible may
HSV, VZV, EBV, HHV-6, and HHV-8. Its activity against CMV be dose-limiting. Other potential adverse effects are nausea, diar-
is up to 100 times greater than that of acyclovir. rhea, fever, rash, headache, insomnia, and peripheral neuropathy.
Ganciclovir is administered intravenously; the bioavailability Central nervous system toxicity (confusion, seizures, psychiatric
of oral ganciclovir is poor, and it is no longer available in the USA. disturbance) and hepatotoxicity have been rarely reported. Intra-
Ganciclovir gel is available for the treatment of acute herpetic ker- vitreal ganciclovir has been associated with vitreous hemorrhage
atitis. Cerebrospinal fluid concentrations are approximately 50% and retinal detachment. Ganciclovir is mutagenic in mammalian
of serum concentrations. The elimination half-life is 4 hours, and cells and carcinogenic and embryotoxic at high doses in animals
the intracellular half-life is prolonged at 16–24 hours. Clearance and causes aspermatogenesis; the clinical significance of these
of the drug is linearly related to creatinine clearance. Ganciclovir preclinical data is unclear.
is readily cleared by hemodialysis. Levels of ganciclovir may rise in patients concurrently taking
Intravenous ganciclovir has been shown to delay progression probenecid or trimethoprim. Concurrent use of ganciclovir with
of CMV retinitis in immunocompromised patients. Dual therapy didanosine may result in increased levels of didanosine.
with foscarnet and ganciclovir is more effective in delaying pro-
gression of retinitis than either drug alone in patients with AIDS
(see Foscarnet), although adverse effects are compounded. Intra- VALGANCICLOVIR
venous ganciclovir is also used to treat CMV colitis, esophagitis,
and pneumonitis (the latter often in combination with intrave- Valganciclovir is an l-valyl ester prodrug of ganciclovir that exists
nous cytomegalovirus immunoglobulin) in immunocompromised as a mixture of two diastereomers. After oral administration, both
patients. Intravenous ganciclovir, followed by either oral ganciclo- diastereomers are rapidly hydrolyzed to ganciclovir by esterases in
vir or high-dose oral acyclovir, reduces the risk of CMV infection the intestinal wall and liver.
in transplant recipients. Limited data in infants with symptomatic Valganciclovir has a bioavailability of 60% and should be
congenital neurologic CMV disease suggest that treatment with taken with food. The AUC 0–24h resulting from oral valganciclovir
IV ganciclovir may reduce hearing loss. The risk of Kaposi’s sar- (900 mg once daily) is similar to that after 5 mg/kg once daily
coma is reduced in AIDS patients receiving long-term ganciclovir, of intravenous ganciclovir and approximately 1.65 times that of
presumably because of activity against HHV-8. oral ganciclovir. The major route of elimination is renal, through
Intravitreal injections of ganciclovir or the intraocular ganci- glomerular filtration and active tubular secretion. Plasma concen-
clovir implant may be used to treat CMV retinitis. Concurrent trations of valganciclovir are reduced ~50% by hemodialysis.
therapy with a systemic anti-CMV agent is necessary to prevent Valganciclovir is as effective as intravenous ganciclovir for the
other sites of end-organ CMV disease. treatment of CMV retinitis and is also indicated for the prevention