Page 886 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 886
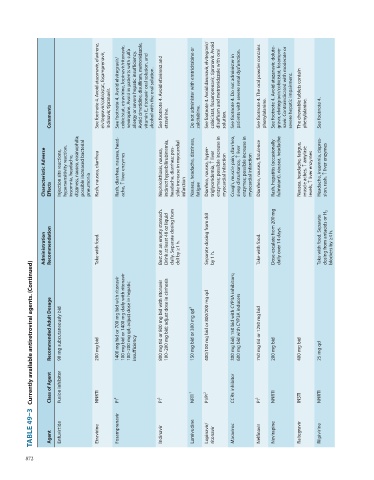
See footnote 4. Avoid atazanavir, efavirenz, elvitegravir/cobicistat, fosamprenavir, See footnote 4. Avoid elvitegravir/ cobicistat, etravirine, lopinavir/ritonavir, nevirapine. Avoid in patients with sulfa allergy or severe hepatic insufficiency. Avoid cimetidine, disulfiram, metronidazole, vitamin E, ritonavir oral solution, and alcohol with the oral solution. See footnote 4. Avoid efavirenz and Do not administer with emtricitabine or See footnote 4. Avoid darunavir, elvitegravir/
Comments indinavir, tipranavir. etravirine. zalcitabine. solution. phenylalanine. severe hepatic impairment. phenylalanine. See footnote 4.
Characteristic Adverse Effects Injection site reactions, hypersensitivity reaction, insomnia, headache, dizziness, nausea, eosinophilia; possible increased bacterial pneumonia Rash, nausea, diarrhea Rash, diarrhea, nausea, head- ache, ↑ liver enzymes Nephrolithiasis, nausea, indirect hyperbilirubinemia, headache, diarrhea; pos- sible increase in myocardial infarction Nausea, headache, dizziness, fatigue Diarrhea, nausea, hyper- triglyceridemia, ↑ liver enzymes; possible increase in myocar
Administration Recommendation Take with food. Best on an empty stomach. Drink at least 48 oz liquid daily. Separate dosing from ddI by ≥1 h. Separate dosing from ddI by 1 h. Take with food. Dose-escalate from 200 mg daily over 14 days. Take with food. Separate dosing from antacids or H 2 blockers by ≥4 h.
Currently available antiretroviral agents. (Continued)
Recommended Adult Dosage 90 mg subcutaneously bid 200 mg bid 1400 mg bid or 700 mg bid with ritonavir 100 mg bid or 1400 mg daily with ritonavir 100–200 mg qd; adjust dose in hepatic insufficiency 800 mg tid or 800 mg bid with ritonavir 100–200 mg bid; adjust dose in cirrhosis 150 mg bid or 300 mg qd 3 400/100 mg bid or 800/200 mg qd 300 mg bid; 150 bid with CYP3A inhibitors; 600 mg bid with CYP3A inducers 750 mg tid or 1250 mg bid 200 mg bid 400 mg bid 25 mg qd
Class of Agent Fusion inhibitor NNRTI PI 2 PI 2 NRTI 1 PI/PI 2 CCR5 inhibitor PI 2 NNRTI INSTI NNRTI
TABLE 49–3 Agent Enfuvirtide Etravirine Fosamprenavir Indinavir Lamivudine Lopinavir/ ritonavir Maraviroc Nelfinavir Nevirapine Raltegravir Rilpivirine
872