Page 60 - March 2020 - WT Site
P. 60
bypassing the primary treatment and increasing the denitrification
Causes/Origin of Possible Corrective
wastewater consequences action volume fail to bring about any improvement, the addition of a
High concentrations of Corrosion in sewers and Avoid blockages in readily degradable substrate (external source of carbon) should
sulphur compounds from tank walls in wastewater the sewerage be considered. Carbon sources for nutrient balancing include: -
chemical and protein treatment plants network Internal C = hydrolysed or acidified primary sludge - External C
processing industries
(meat and poultry Neighbours suffer odour Add iron salts to = industrial residues (from breweries, dairies, sugar industry) and
processing) nuisance the sewer (e.g. at industrial products (methanol, ethanol, acetic acid).
Anaerobic processes in Increased growth of the pumping
the sewerage system, sulphur oxidising stations) COD:BOD ratio
which cause sulphur filamentous 5
compounds to be reduced bacteria (Type 021 N) The ratio of these two sum parameters is a measure of the
to hydrogen sulphide biodegradability of the wastewater pollution load. If the
Table 2: Causes and Effects of High Sulphur Concentrations COD:BOD ratio does not exceed 2:1, the biodegradability is
5
said to be good. Higher values indicate the presence of poorly
to vary widely in its composition. Experience has shown that
the C:N:P ratio in municipal wastewater is about 100:20:5. The biodegradable substances.
excess N and P compounds can usually be eliminated from the Example: A municipal wastewater treatment plant with a high
wastewater without any great difficulty using modern methods. proportion of industrial wastewater has the following nutrient
parameters in the inflow to the biological treatment stage (Table 5).
If the wastewater in the inflow to the biological stage is deficient
in one of the main nutrients, a wide range of problems may The BOD :N ratio of 2.45 is too low for adequate denitrification
5
occur (Table 3). For efficient denitrification, a certain proportion to occur. External carbon compounds should therefore be added.
of readily biodegradable C compounds must be present. After However, a number of calculations have to be carried out before
municipal wastewater has passed through the primary settling this is done:
tank, it has a BOD5:N ratio of 100:25 (=5). If the ratio falls
below 100:40 (=2.5), the efficiency of the denitrification process 1. Amount of nitrogen that is not to be denitrified (∑Nn.z.d.):
is impaired, resulting in higher nitrate values in the outflow. If ¾ see Table 6
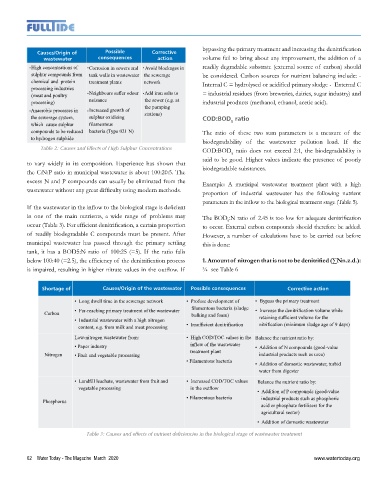
Shortage of
Causes/Origin of the wastewater Possible consequences Corrective action
• Long dwell time in the sewerage network • Profuse development of • Bypass the primary treatment
Carbon • Far-reaching primary treatment of the wastewater filamentous bacteria (sludge • Increase the denitrification volume while
bulking and foam)
• Industrial wastewater with a high nitrogen retaining sufficient volume for the
content, e.g. from milk and meat processing • Insufficient denitrification nitrification (minimum sludge age of 9 days)
Low-nitrogen wastewater from: • High COD/TOC values in the Balance the nutrient ratio by:
• Paper industry inflow of the wastewater • Addition of N compounds (good-value
Nitrogen • Fruit and vegetable processing treatment plant industrial products such as urea)
• Filamentous bacteria
• Addition of domestic wastewater, turbid
water from digester
• Landfill leachate, wastewater from fruit and • Increased COD/TOC values Balance the nutrient ratio by:
vegetable processing in the outflow
• Addition of P compounds (good-value
• Filamentous bacteria industrial products such as phosphoric
Phosphorus
acid or phosphate fertilisers for the
agricultural sector)
• Addition of domestic wastewater
Table 3: Causes and effects of nutrient deficiencies in the biological stage of wastewater treatment
62 Water Today - The Magazine March 2020