Page 12 - E-Science Prep1 2024-2025 t2 Unit1_Neat - Copy
P. 12
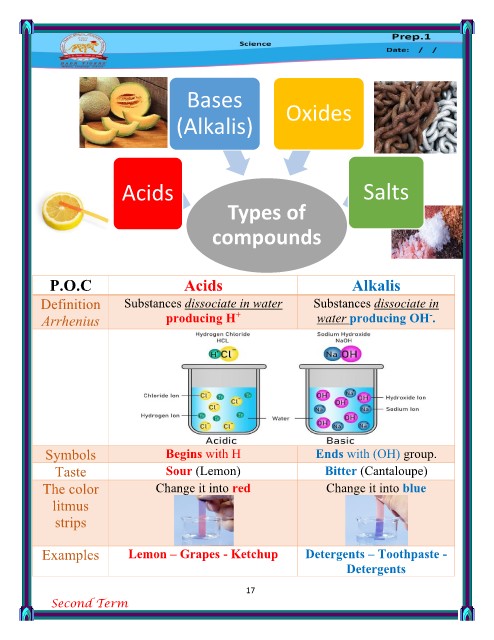
P.O.C Acids Alkalis
Name Its name is related to the name Its name is related to the name
of anion composes it. of cation composes it except
Chemical
formula Anion of non-metal except O H
Anion of atomic gp. except OH
Total charge 1- Begins with cation
1- Begins with H+ 2- Ends with (OH)-
2- The symbol of anion hydroxide.
element / atomic gp. 3- The number of OH gp.
3- The number of H atoms = = the magnitude of
cation charges
the magnitude of anion
charges Zero
Zero
Ca(OH)2 No. of hydroxide gp.
HBr NaOH more than 1 is between
H2SO3 Mg(OH)2
H3PO4 NH4OH brackets.
P.O.C Acids don’t have O Acids have O in atomic gp.
Steps of (Oxyacid)
naming
1- Begins “Prefix” Hydro 1- Begins anion name
Suffix of
acids 2- Anion name “atomic gp.”
Examples “non-metal / atomic gp.” 2- Acid
3- Acid
“ide” of anion is replaced by “ate” of anion is replace by
“ic” in acid “ic” in acid
“ite” of anion is replace by
“ous” in acid
HCl Hydrochloric acid H2SO4 Sulphuric acid
HBr Hydrobromic acid H2SO3 Sulphurous acid
H2S Hydrosulphuric acid HNO3
HNO2 Nitric acid
H3PO4 Nitrous acid
Phosphoric acid
18
Second Term