Page 15 - E-Science Prep1 2024-2025 t2 Unit1_Neat - Copy
P. 15
Metallic / non-metallic element burns in presence of oxygen
producing oxides
P.O.C Metal oxides Non-metal oxides
Basic oxides Acidic oxides
Definition Oxides produced from Oxides produced from
Applications burning of metals in burning of non-metals in
oxygen oxygen
Basic oxides: Acidic oxides:
Some metal oxides dissolve Non-metal oxides dissolve
in water forming alkali in water forming acids
Mg burns in oxygen forming S burns in oxygen forming
Magnesium oxide Sulphur trioxide
Dissolves in water forming Dissolves in water forming
Magnesium hydroxide Sulphuric acid
Turns red litmus strip to blue Turns blue litmus strip to red
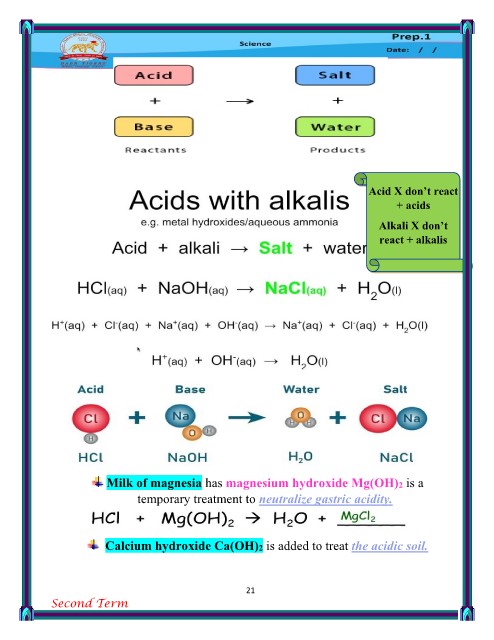
All alkalis are bases but not all Metal oxides v Metal oxides X
bases are alkalis. react + acids
don’t react + alkalis
Basic / metal oxides that dissolve Non-metal oxides
v react + alkalis Non-metal oxides
in water are alkalis.
X don’t react + acids
22
Second Term