Page 33 - Finalll Science BB Prep2 t1 2025_Neat
P. 33
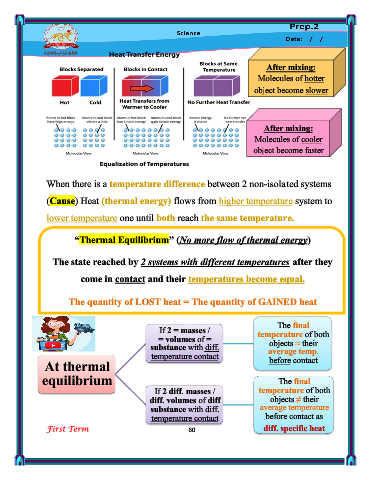
After mixing:
Molecules of hotter
object become slower
After mixing:
Molecules of cooler
object become faster
When there is a temperature difference between 2 non-isolated systems
(Cause) Heat (thermal energy) flows from higher temperature system to
lower temperature one until both reach the same temperature.
“Thermal Equilibrium” (No more flow of thermal energy)
The state reached by 2 systems with different temperatures after they
come in contact and their temperatures become equal.
The quantity of LOST heat = The quantity of GAINED heat
The final
If 2 = masses /
= volumes of = temperature of both
objects ≈ their
substance with diff. average temp.
temperature contact
At thermal before contact
equilibrium The final
If 2 diff. masses / temperature of both
diff. volumes of diff objects ≠ their
substance with diff. average temperature
temperature contact before contact as
First Term 60 diff. specific heat