Page 34 - Org 3 theoritical book 2024-25
P. 34
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
Electrophilic substitution reactions:
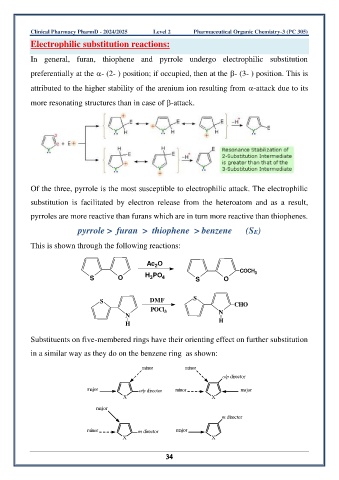
In general, furan, thiophene and pyrrole undergo electrophilic substitution
preferentially at the - (2- ) position; if occupied, then at the - (3- ) position. This is
attributed to the higher stability of the arenium ion resulting from -attack due to its
more resonating structures than in case of -attack.
Of the three, pyrrole is the most susceptible to electrophilic attack. The electrophilic
substitution is facilitated by electron release from the heteroatom and as a result,
pyrroles are more reactive than furans which are in turn more reactive than thiophenes.
pyrrole > furan > thiophene > benzene (SE)
This is shown through the following reactions:
Ac O
2
COCH 3
S O H PO 4 S O
3
S DMF S CHO
POCl 3 N
N
H H
Substituents on five-membered rings have their orienting effect on further substitution
in a similar way as they do on the benzene ring as shown:
minor minor
o/p director
major o/p director minor major
X X
major
m director
minor m director major
X X