Page 36 - Org 3 theoritical book 2024-25
P. 36
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
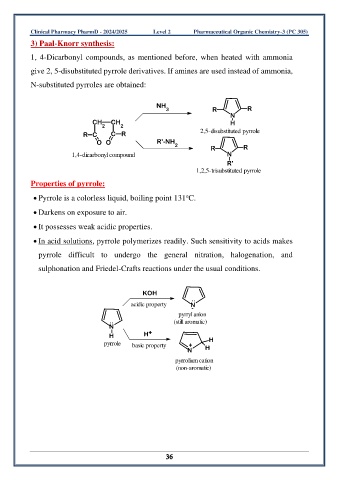
3) Paal-Knorr synthesis:
1, 4-Dicarbonyl compounds, as mentioned before, when heated with ammonia
give 2, 5-disubstituted pyrrole derivatives. If amines are used instead of ammonia,
N-substituted pyrroles are obtained:
NH
3 R R
N
CH CH H
2 2
R C C R 2,5-disubstituted pyrrole
O O R'-NH 2
R R
1,4-dicarbonyl compound N
R'
1,2,5-trisubstituted pyrrole
Properties of pyrrole:
o
• Pyrrole is a colorless liquid, boiling point 131 C.
• Darkens on exposure to air.
• It possesses weak acidic properties.
• In acid solutions, pyrrole polymerizes readily. Such sensitivity to acids makes
pyrrole difficult to undergo the general nitration, halogenation, and
sulphonation and Friedel-Crafts reactions under the usual conditions.
KOH
..
acidic property N -
-
H
pyrryl anion cyclopentadienyl anion
.. (still aromatic)
N
H H +
pyrrole basic property + H
N H
pyrrolium cation
(non-aromatic)