Page 41 - Org 3 theoritical book 2024-25
P. 41
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
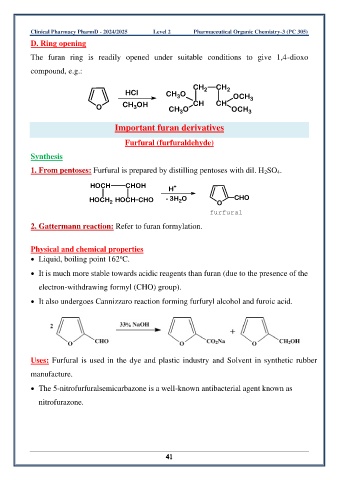
D. Ring opening
The furan ring is readily opened under suitable conditions to give 1,4-dioxo
compound, e.g.:
CH CH
HCl CH O 2 2 OCH 3
3
O CH OH CH O CH CH OCH 3
3
3
Important furan derivatives
Furfural (furfuraldehyde)
Synthesis
1. From pentoses: Furfural is prepared by distilling pentoses with dil. H 2SO 4.
HO CH CHOH H +
HOCH 2 HO CH CHO - 3H O O CHO
2
furfural
2. Gattermann reaction: Refer to furan formylation.
Physical and chemical properties
• Liquid, boiling point 162 C.
o
• It is much more stable towards acidic reagents than furan (due to the presence of the
electron-withdrawing formyl (CHO) group).
• It also undergoes Cannizzaro reaction forming furfuryl alcohol and furoic acid.
Uses: Furfural is used in the dye and plastic industry and Solvent in synthetic rubber
manufacture.
• The 5-nitrofurfuralsemicarbazone is a well-known antibacterial agent known as
nitrofurazone.