Page 43 - Org 3 theoritical book 2024-25
P. 43
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
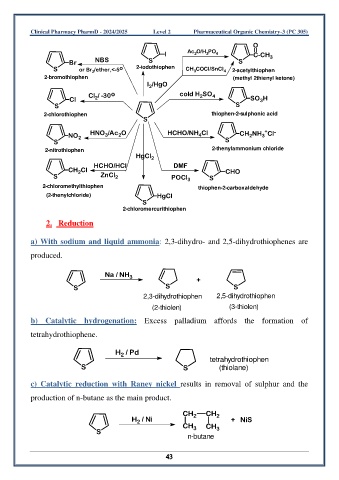
O
I Ac O/H PO 4 C-CH 3
2
3
Br NBS S S
S or Br /ether,<-5 o 2-iodothiophen CH COCl/SnCl 4 2-acetylthiophen
3
2
2-bromothiophen (methyl 2thienyl ketone)
I /HgO
2
Cl / -30 o cold H SO 4
2
Cl 2 SO H
3
S S
2-chlorothiophen thiophen-2-sulphonic acid
S
+
NO 2 HNO /Ac O HCHO/NH Cl CH NH Cl -
4
2
3
3
2
S S
2-nitrothiophen 2-thenylammonium chloride
HgCl 2
HCHO/HCl DMF
CH Cl CHO
2
S ZnCl 2 POCl 3 S
2-chloromethylthiophen thiophen-2-carboxaldehyde
(2-thenylchloride) HgCl
S
2-chloromercurithiophen
2. Reduction
a) With sodium and liquid ammonia: 2,3-dihydro- and 2,5-dihydrothiophenes are
produced.
Na / NH 3 +
S S S
2,3-dihydrothiophen 2,5-dihydrothiophen
(2-thiolen) (3-thiolen)
b) Catalytic hydrogenation: Excess palladium affords the formation of
tetrahydrothiophene.
H / Pd
2
tetrahydrothiophen
S S (thiolane)
c) Catalytic reduction with Raney nickel results in removal of sulphur and the
production of n-butane as the main product.
CH 2 CH 2
H / Ni + NiS
2
CH 3 CH 3
S
n-butane