Page 56 - Org 3 theoritical book 2024-25
P. 56
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
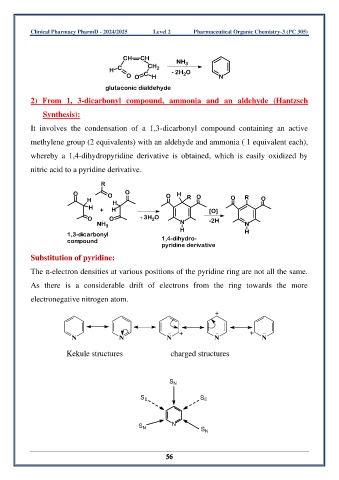
CH CH NH 3
H C CH 2 - 2H O
O O C H 2 N
glutaconic dialdehyde
2) From 1, 3-dicarbonyl compound, ammonia and an aldehyde (Hantzsch
Synthesis):
It involves the condensation of a 1,3-dicarbonyl compound containing an active
methylene group (2 equivalents) with an aldehyde and ammonia ( 1 equivalent each),
whereby a 1,4-dihydropyridine derivative is obtained, which is easily oxidized by
nitric acid to a pyridine derivative.
R
O O O O H
H H R O O R O
H + H [O]
O O - 3H O -2H
2
NH 3 N N
1,3-dicarbonyl H H
compound 1,4-dihydro-
pyridine derivative
Substitution of pyridine:
The π-electron densities at various positions of the pyridine ring are not all the same.
As there is a considerable drift of electrons from the ring towards the more
electronegative nitrogen atom.
+
+ +
N N N N N
Kekule structures charged structures
S N
S E S E
S N N S N