Page 53 - Org 3 theoritical book 2024-25
P. 53
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
Imidazole is a weak base, but it is more basic than pyrazole and pyrrole. It forms
stable salt with mineral acids.
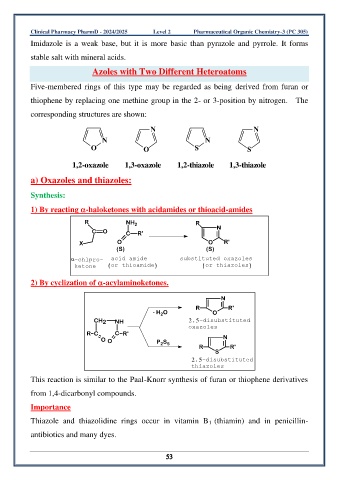
Azoles with Two Different Heteroatoms
Five-membered rings of this type may be regarded as being derived from furan or
thiophene by replacing one methine group in the 2- or 3-position by nitrogen. The
corresponding structures are shown:
N N
N N
O O S S
1,2-oxazole 1,3-oxazole 1,2-thiazole 1,3-thiazole
a) Oxazoles and thiazoles:
Synthesis:
1) By reacting -haloketones with acidamides or thioacid-amides
R NH 2 R
C O C R' N
X O O R'
(S) (S)
-chlpro- acid amide substituted oxazoles
ketone (or thioamide) (or thiazoles)
2) By cyclization of -acylaminoketones.
N
R R'
- H O O
2
CH 2 NH 2,5-disubstituted
oxazoles
R C C R'
O O P S N
2 5
R R'
S
2,5-disubstituted
thiazoles
This reaction is similar to the Paal-Knorr synthesis of furan or thiophene derivatives
from 1,4-dicarbonyl compounds.
Importance
Thiazole and thiazolidine rings occur in vitamin B 1 (thiamin) and in penicillin-
antibiotics and many dyes.