Page 33 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 33
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
Five-Membered Heterocycles
1. Compounds with One Heteroatom
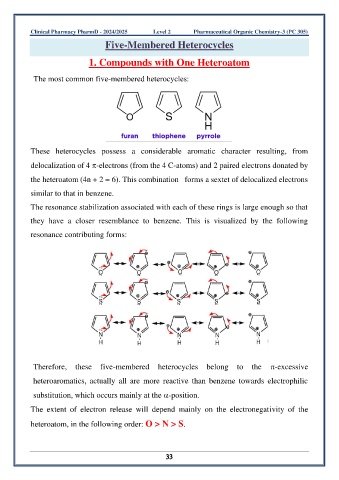
The most common five-membered heterocycles:
These heterocycles possess a considerable aromatic character resulting, from
delocalization of 4 -electrons (from the 4 C-atoms) and 2 paired electrons donated by
the heteroatom (4n + 2 = 6). This combination forms a sextet of delocalized electrons
similar to that in benzene.
The resonance stabilization associated with each of these rings is large enough so that
they have a closer resemblance to benzene. This is visualized by the following
resonance contributing forms:
Therefore, these five-membered heterocycles belong to the -excessive
heteroaromatics, actually all are more reactive than benzene towards electrophilic
substitution, which occurs mainly at the -position.
The extent of electron release will depend mainly on the electronegativity of the
heteroatom, in the following order: O > N > S.