Page 46 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 46
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
Fused Heterocyclic Systems of Pyrrole, Furan
and Thiophen with Benzene
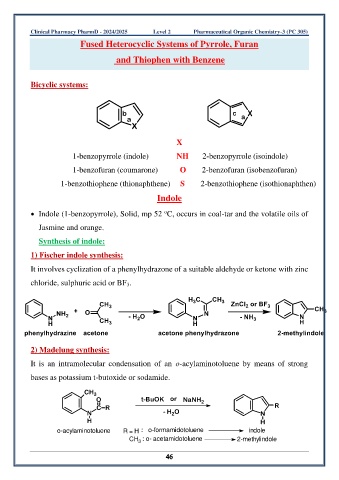
Bicyclic systems:
b c X
a a
X
X
1-benzopyrrole (indole) NH 2-benzopyrrole (isoindole)
1-benzofuran (coumarone) O 2-benzofuran (isobenzofuran)
1-benzothiophene (thionaphthene) S 2-benzothiophene (isothionaphthen)
Indole
o
• Indole (1-benzopyrrole), Solid, mp 52 C, occurs in coal-tar and the volatile oils of
Jasmine and orange.
Synthesis of indole:
1) Fischer indole synthesis:
It involves cyclization of a phenylhydrazone of a suitable aldehyde or ketone with zinc
chloride, sulphuric acid or BF 3.
2) Madelung synthesis:
It is an intramolecular condensation of an o-acylaminotoluene by means of strong
bases as potassium t-butoxide or sodamide.
CH 3
O t-BuOK or NaNH 2
C R R
N - H O N
2
H H
o-acylaminotoluene R = H : o-formamidotoluene indole
CH : o- acetamidotoluene 2-methylindole
3