Page 43 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 43
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
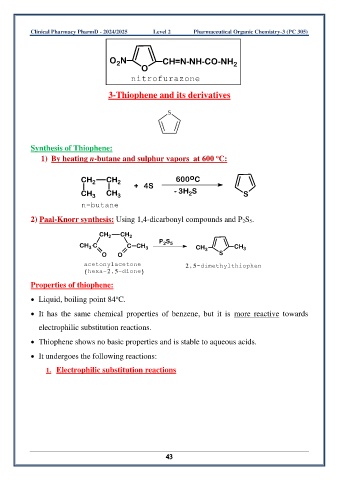
O N CH=N-NH-CO-NH
2
O 2
nitrofurazone
3-Thiophene and its derivatives
Synthesis of Thiophene:
o
1) By heating n-butane and sulphur vapors at 600 C:
o
CH 2 CH 2 600 C
+ 4S
CH 3 CH 3 - 3H S S
2
n-butane
2) Paal-Knorr synthesis: Using 1,4-dicarbonyl compounds and P 2S 5.
CH 2 CH 2
2 5
CH 3 C C CH 3 P S CH 3 CH 3
O O S
acetonylacetone 2,5-dimethylthiophen
(hexa-2,5-dione)
Properties of thiophene:
o
• Liquid, boiling point 84 C.
• It has the same chemical properties of benzene, but it is more reactive towards
electrophilic substitution reactions.
• Thiophene shows no basic properties and is stable to aqueous acids.
• It undergoes the following reactions:
1. Electrophilic substitution reactions