Page 68 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 68
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
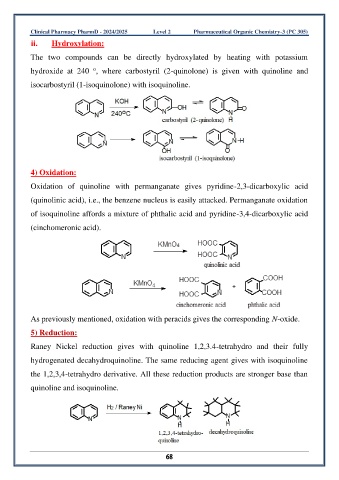
ii. Hydroxylation:
The two compounds can be directly hydroxylated by heating with potassium
o
hydroxide at 240 , where carbostyril (2-quinolone) is given with quinoline and
isocarbostyril (1-isoquinolone) with isoquinoline.
4) Oxidation:
Oxidation of quinoline with permanganate gives pyridine-2,3-dicarboxylic acid
(quinolinic acid), i.e., the benzene nucleus is easily attacked. Permanganate oxidation
of isoquinoline affords a mixture of phthalic acid and pyridine-3,4-dicarboxylic acid
(cinchomeronic acid).
As previously mentioned, oxidation with peracids gives the corresponding N-oxide.
5) Reduction:
Raney Nickel reduction gives with quinoline 1,2,3.4-tetrahydro and their fully
hydrogenated decahydroquinoline. The same reducing agent gives with isoquinoline
the 1,2,3,4-tetrahydro derivative. All these reduction products are stronger base than
quinoline and isoquinoline.