Page 64 - Pharmaceutical Organic Chemmistry-3 (Theoritical book) 24-25
P. 64
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
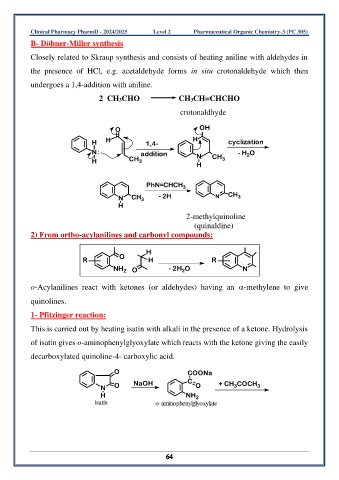
B- Döbner-Miller synthesis
Closely related to Skraup synthesis and consists of heating aniline with aldehydes in
the presence of HCl, e.g. acetaldehyde forms in situ crotonaldehyde which then
undergoes a 1,4-addition with aniline.
2 CH3CHO CH3CH=CHCHO
crotonaldhyde
O OH
H H 1,4- H cyclization
N: addition N - H O
2
H CH 3 CH 3
H
PhN=CHCH 3
N CH 3 - 2H N CH 3
H
2-methylquinoline
(quinaldine)
2) From ortho-acylanilines and carbonyl compounds:
H
O
R H R
NH 2 O - 2H O N
2
o-Acylanilines react with ketones (or aldehydes) having an -methylene to give
quinolines.
1- Pfitzinger reaction:
This is carried out by heating isatin with alkali in the presence of a ketone. Hydrolysis
of isatin gives o-aminophenylglyoxylate which reacts with the ketone giving the easily
decarboxylated quinoline-4- carboxylic acid.
O COONa
3
N O NaOH C O + CH COCH 3
H NH 2
isatin o-aminophenylglyoxylate